1. The document contains 50 multiple choice questions from a 1997 chemistry exam.
2. The questions cover a wide range of chemistry topics including organic chemistry, inorganic chemistry, physical chemistry, and thermodynamics.
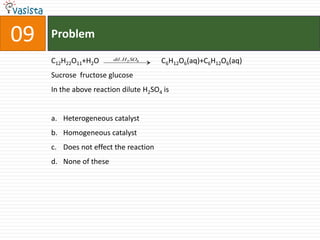
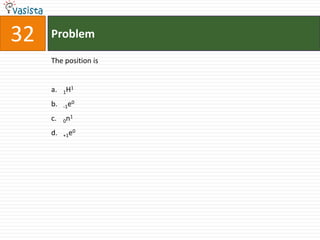
3. Multiple answer choices are provided for each question, with one being the correct answer.




















![Problem20Which of the following has square planar structure?[Ni(CN)4]2-NiC14]2-[Cr(CN)6]3-[Ni(CO)4]](https://image.slidesharecdn.com/chemistry-1997-111007054939-phpapp02/85/AFMC-Chemistry-1997-22-320.jpg)
![Problem21Which of the following is paramagnetic?[Ni(CN)4]2-[Ni(CO)4]NiC14]2-[Co(NH3)6]3+](https://image.slidesharecdn.com/chemistry-1997-111007054939-phpapp02/85/AFMC-Chemistry-1997-23-320.jpg)

























![Problem48Which of the following is ferroelectric compound/Pb2O3BaTiO3PbZrO3K4[Fe(CN)6]](https://image.slidesharecdn.com/chemistry-1997-111007054939-phpapp02/85/AFMC-Chemistry-1997-50-320.jpg)


