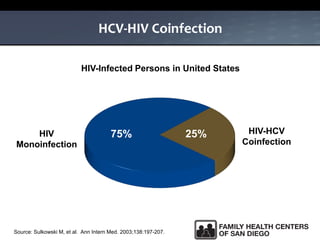
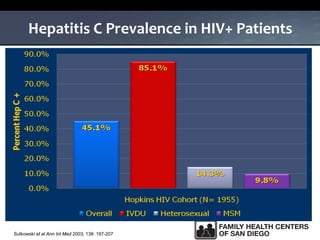
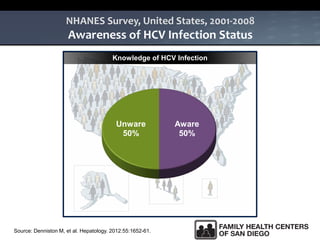
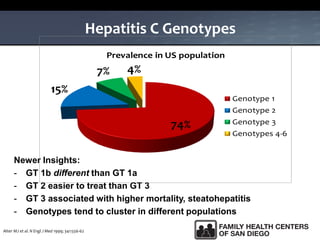
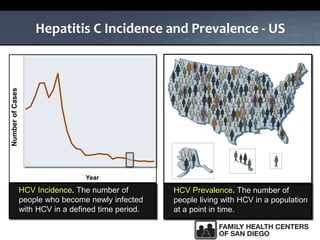
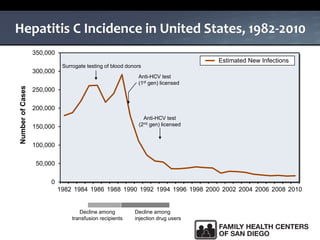
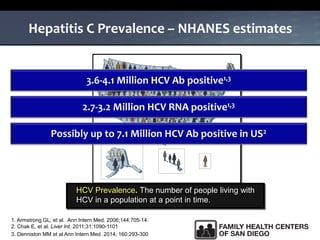
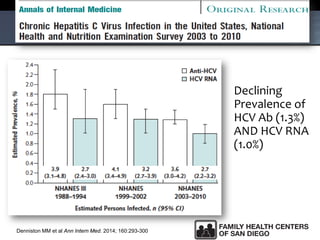
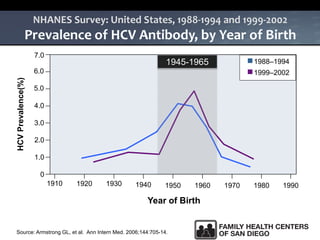
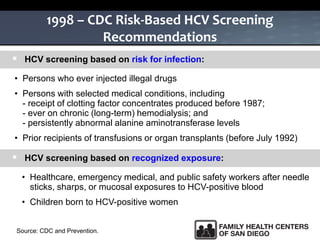
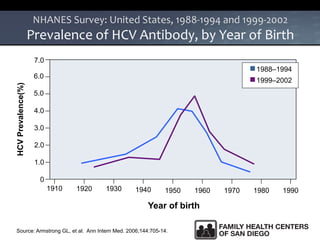
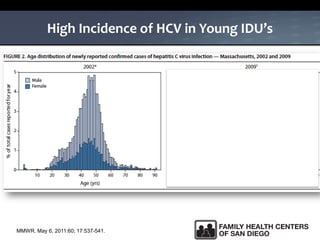
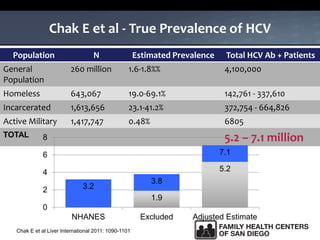
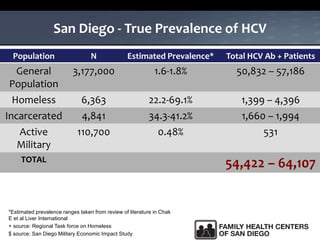
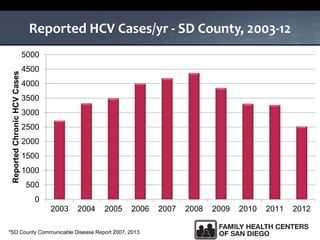
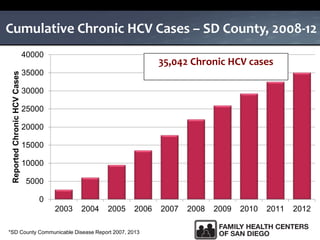
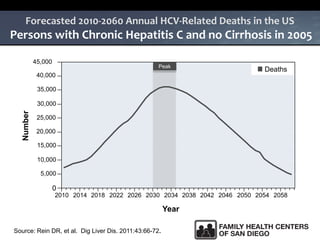
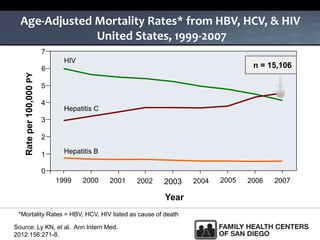
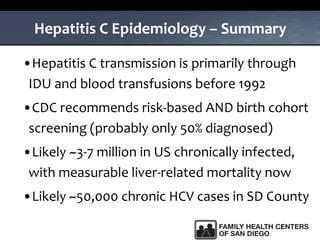
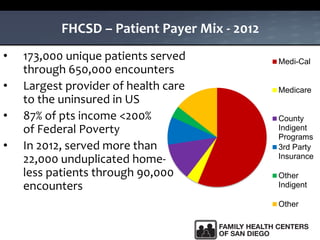
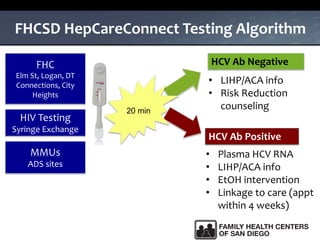
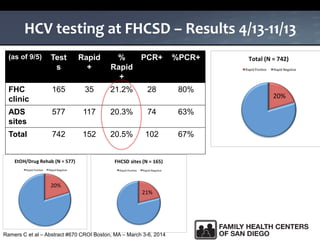
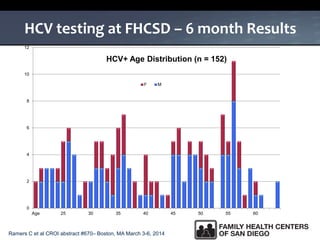
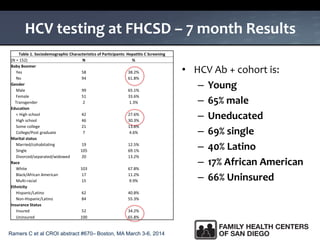
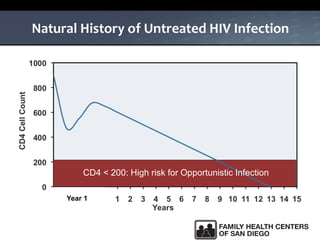
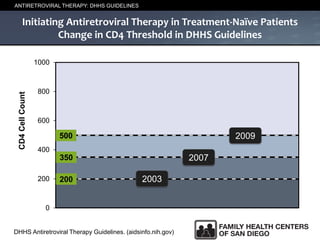
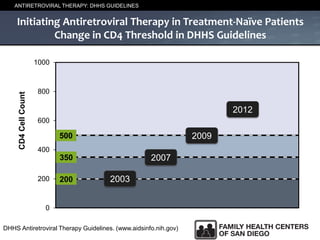
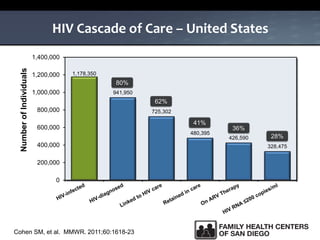
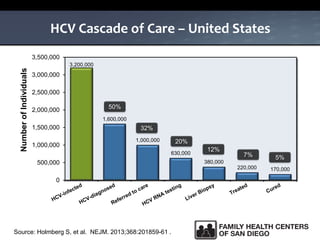
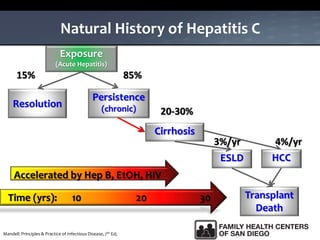
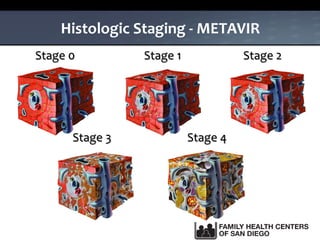
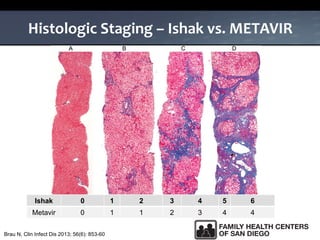
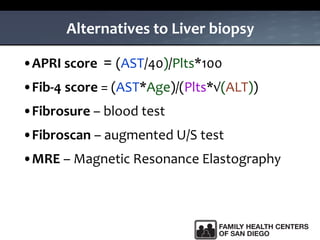
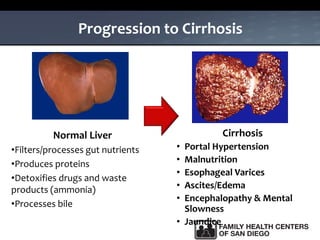
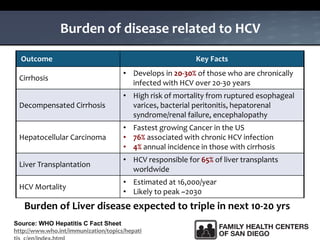
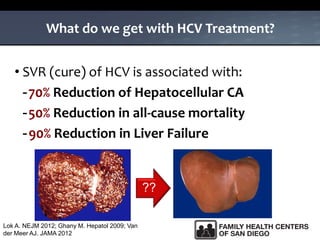
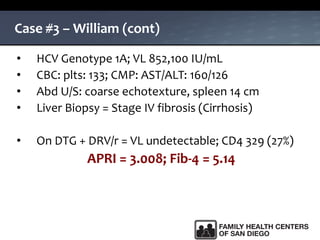
The UC San Diego Antiviral Research Center hosts weekly presentations to share the latest research and clinical practices related to various infectious diseases, including HIV and Hepatitis C (HCV). The document highlights key findings on HCV epidemiology, transmission risks, and screening recommendations, especially focused on community health settings. It also discusses the significant burden of HCV-related mortality and the urgent need for effective screening and treatment strategies in the San Diego region.