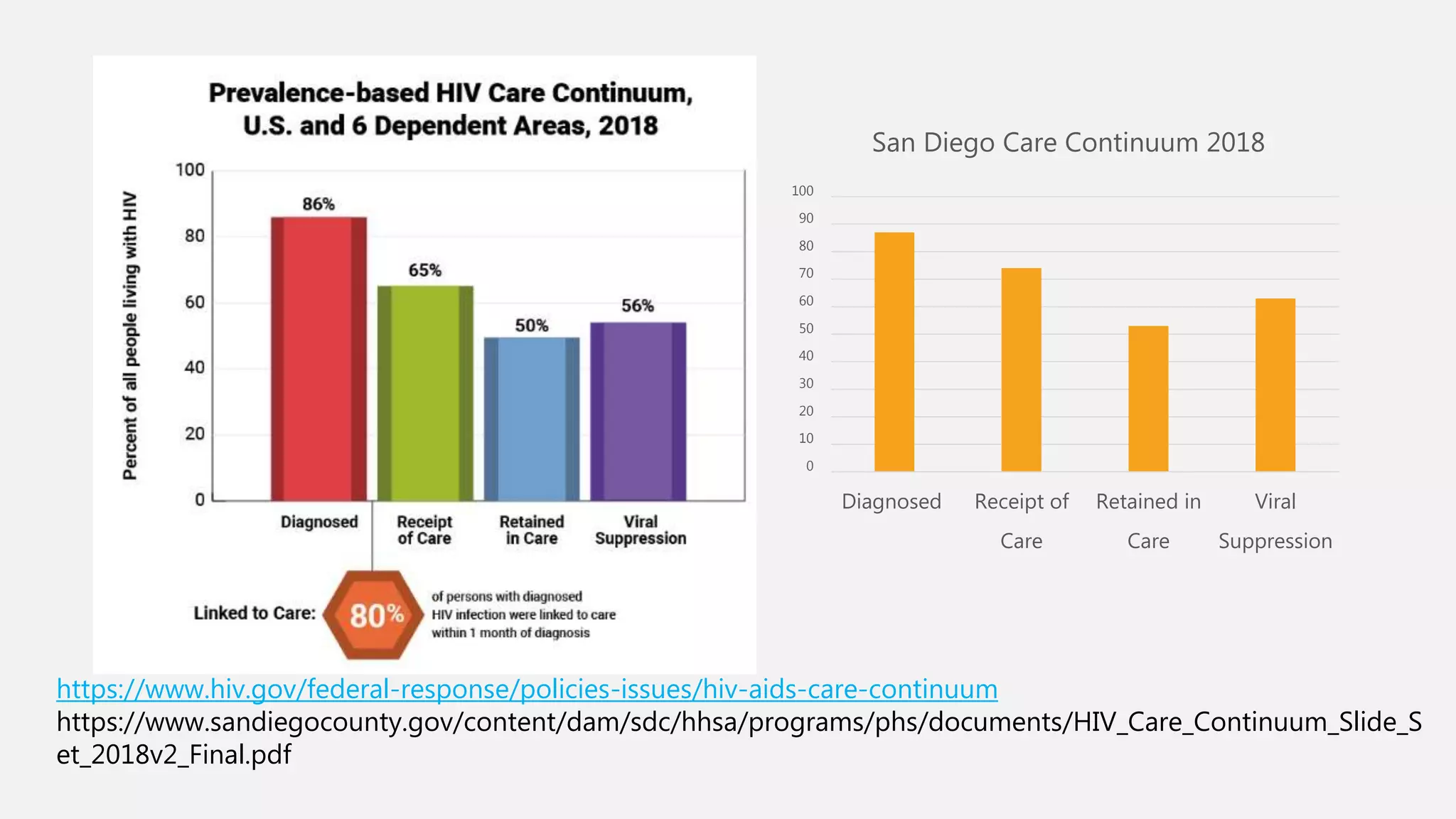
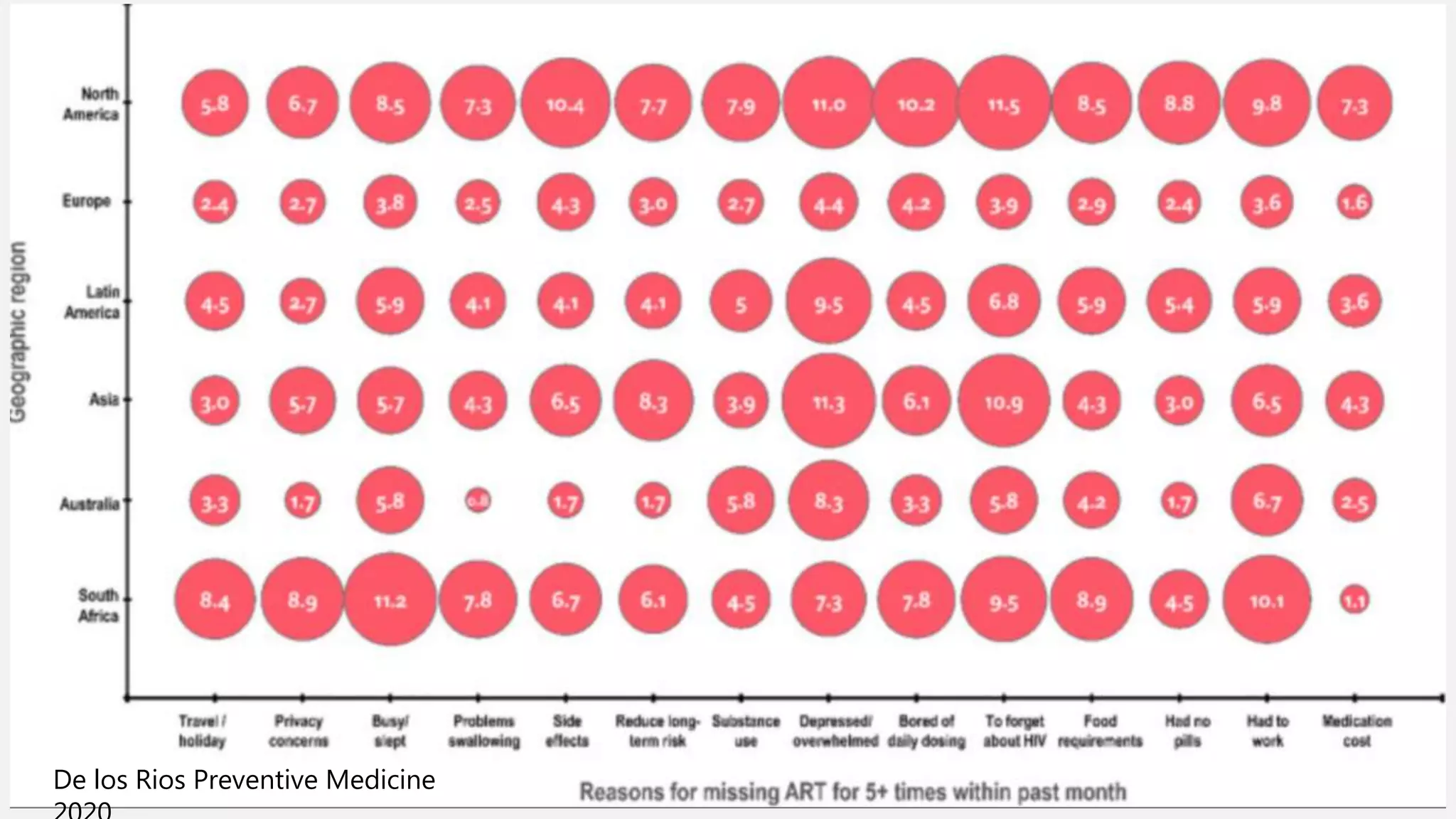
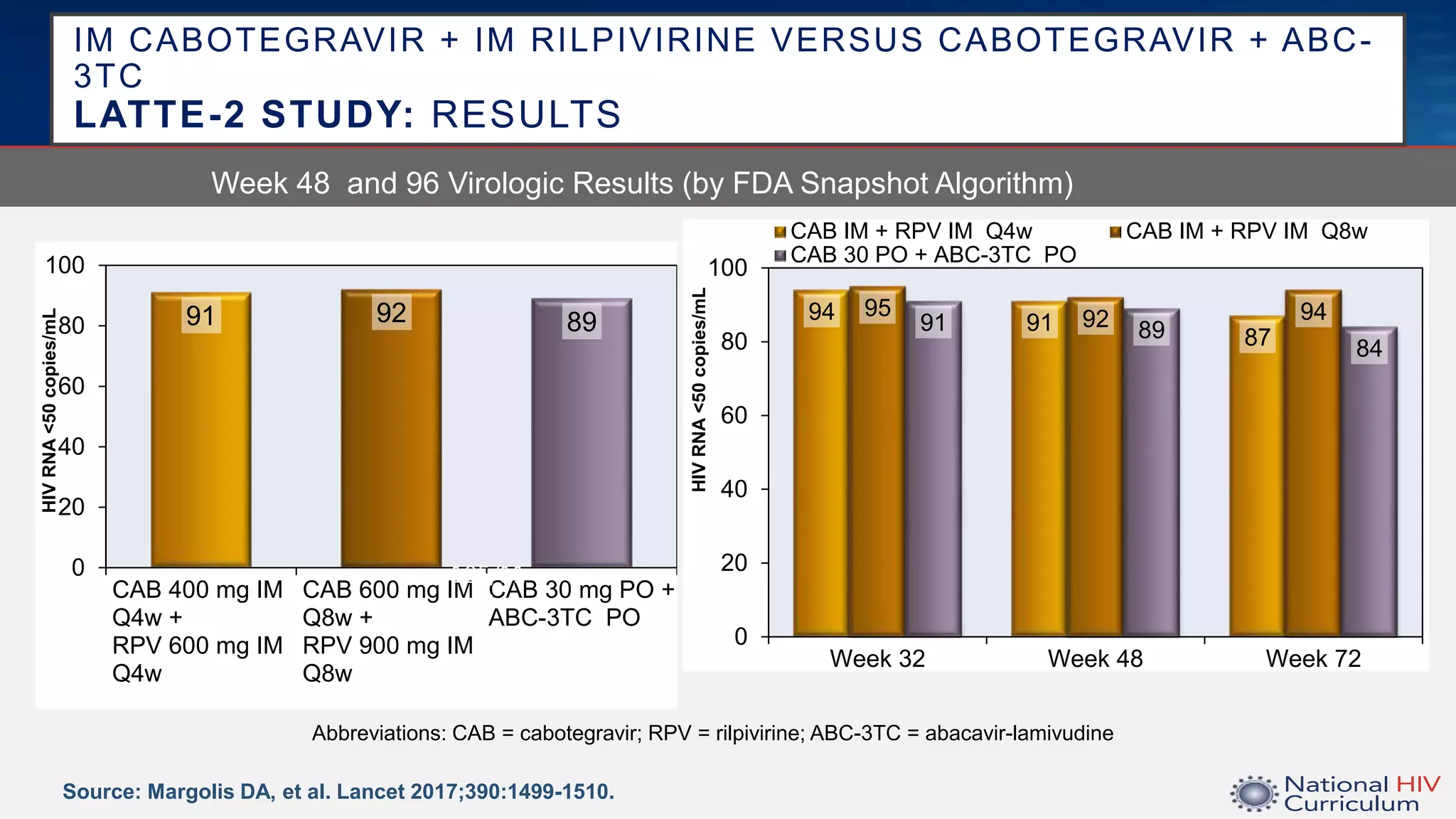
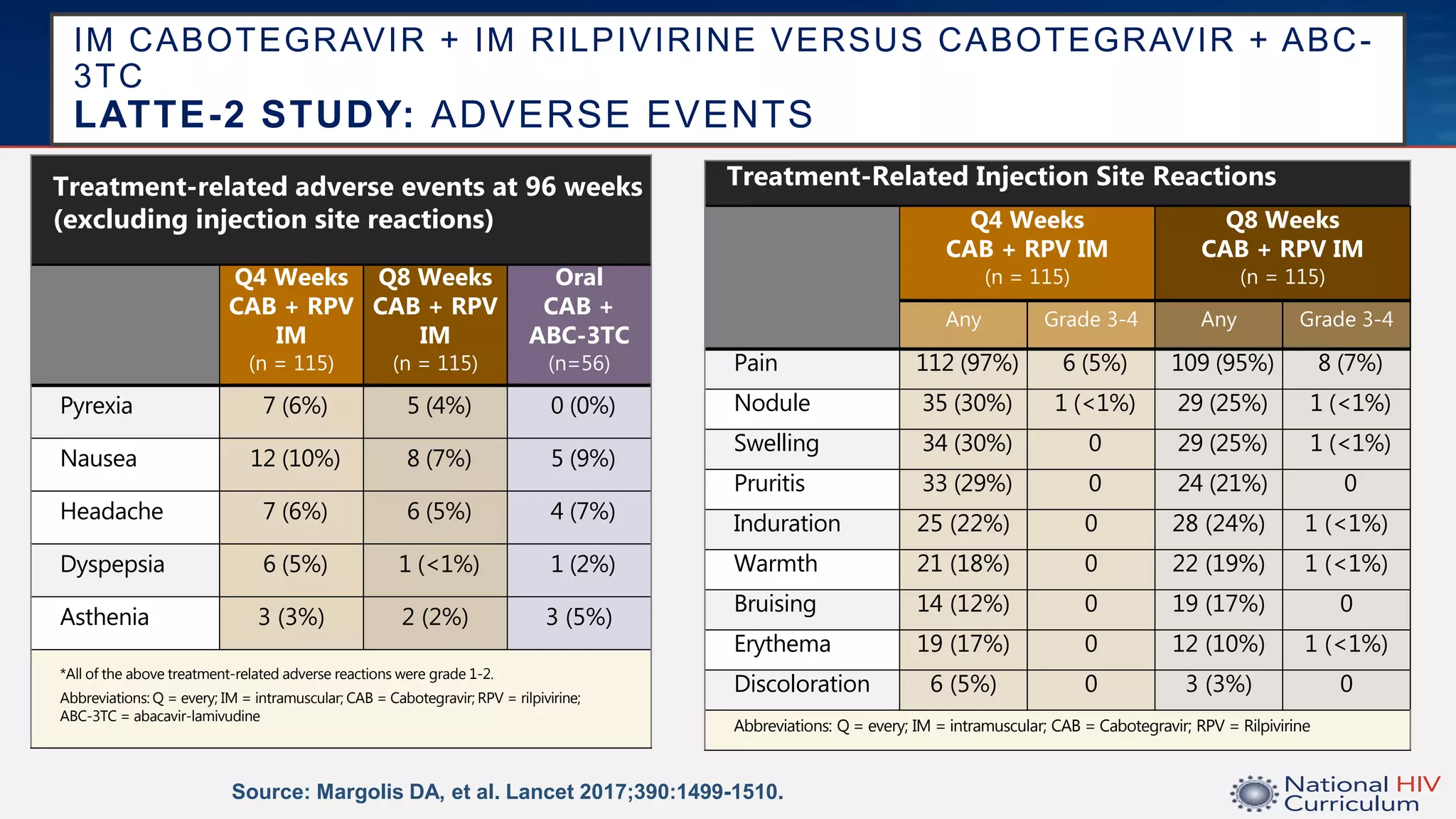
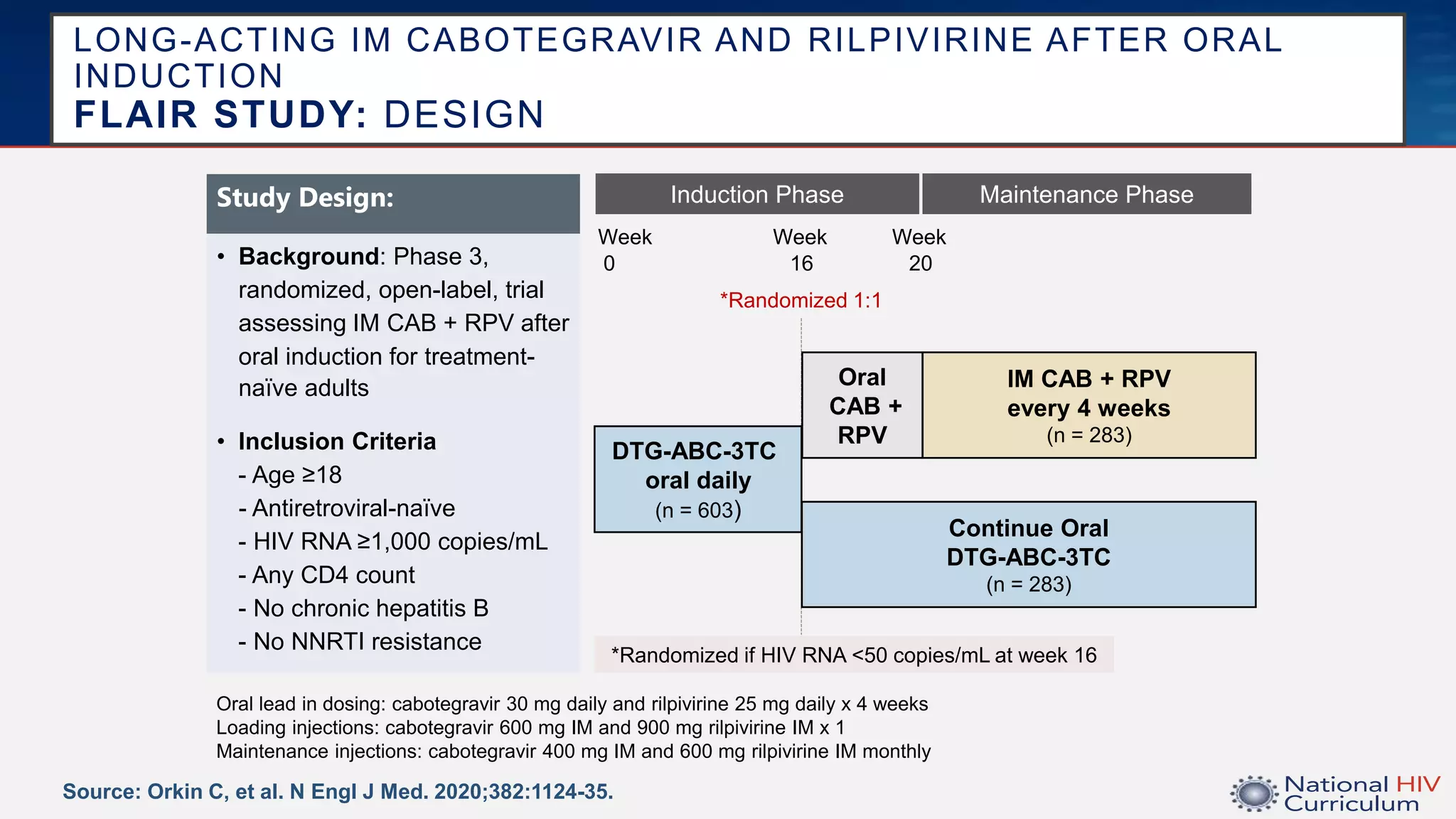
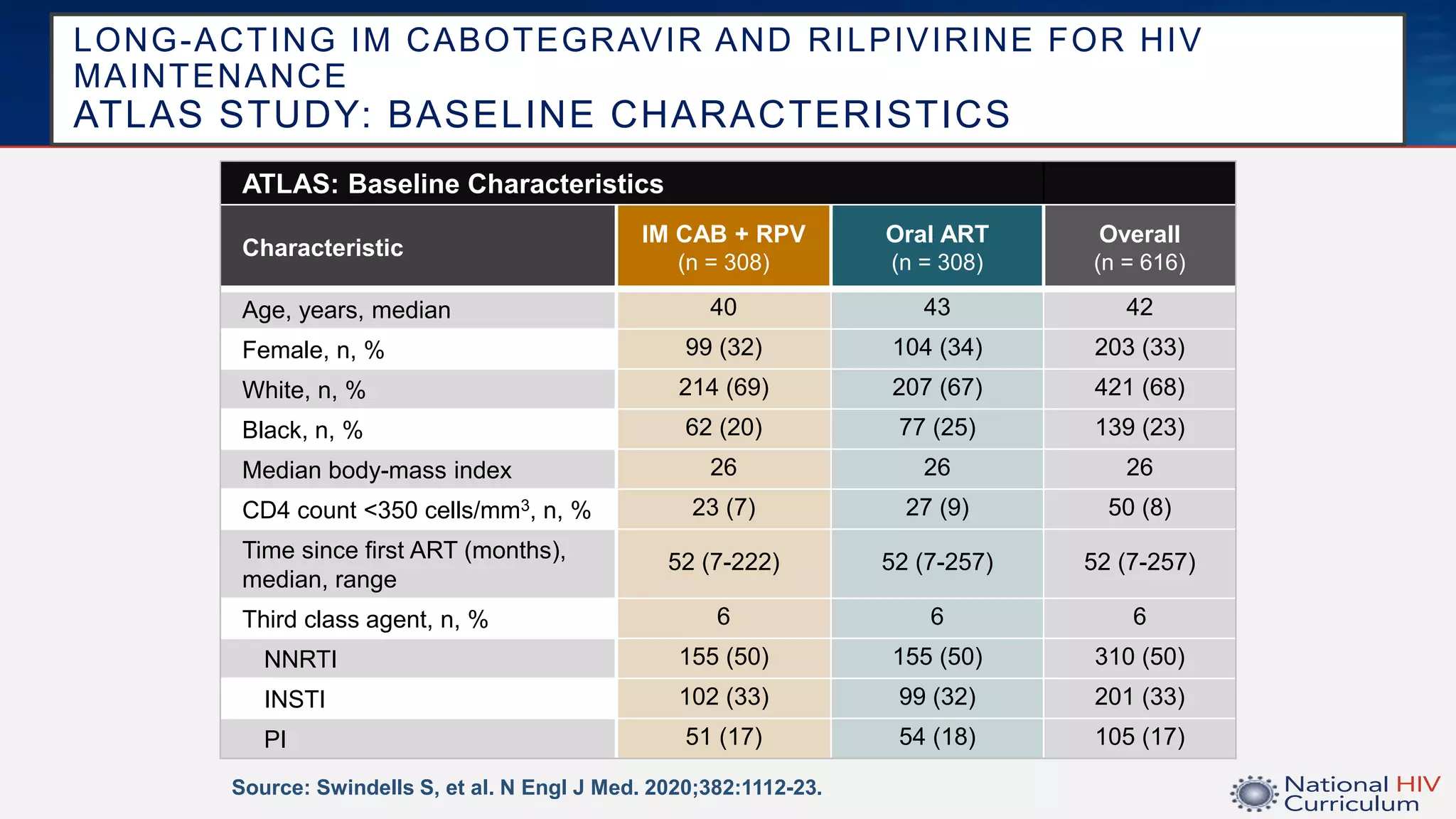
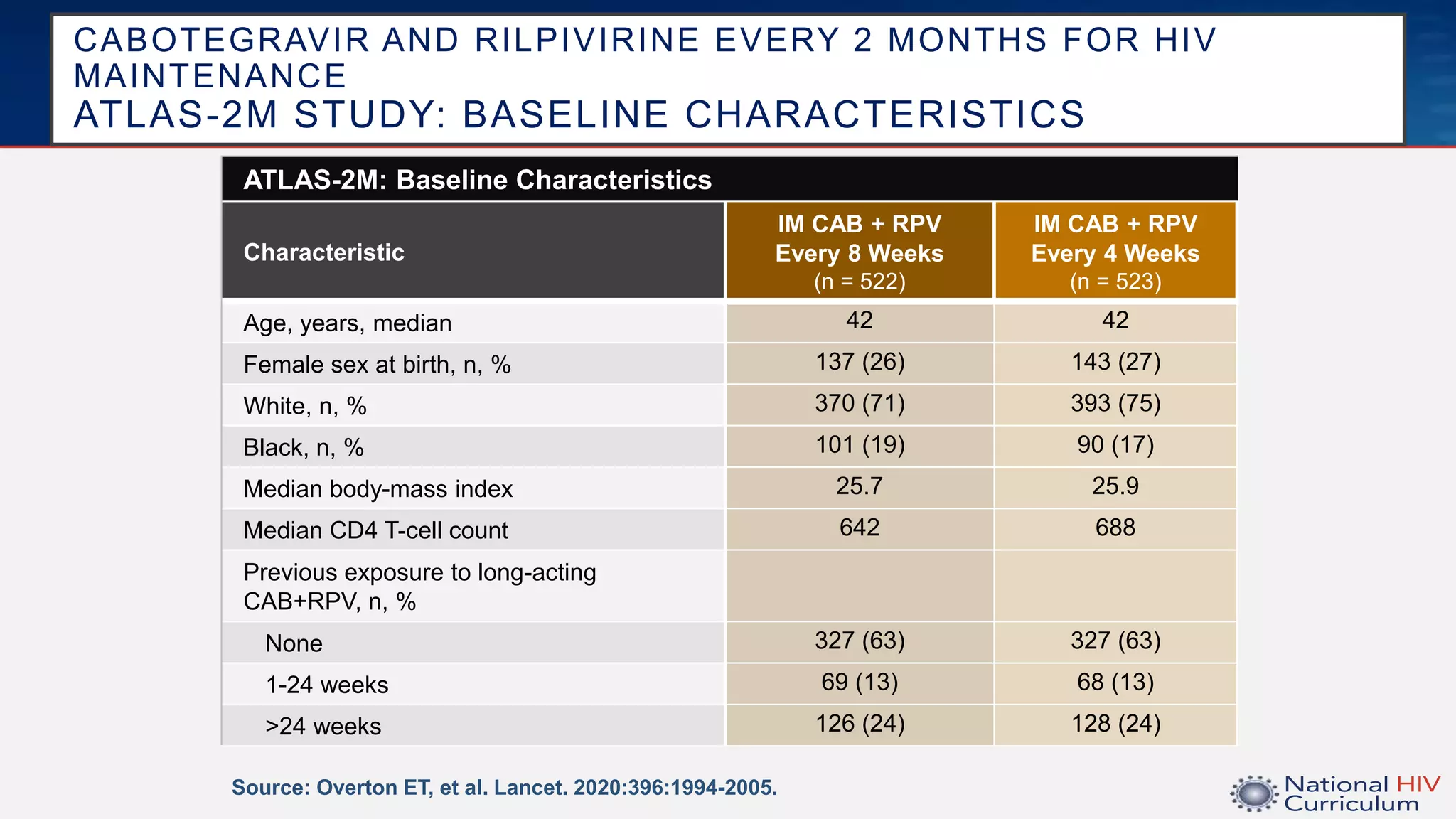
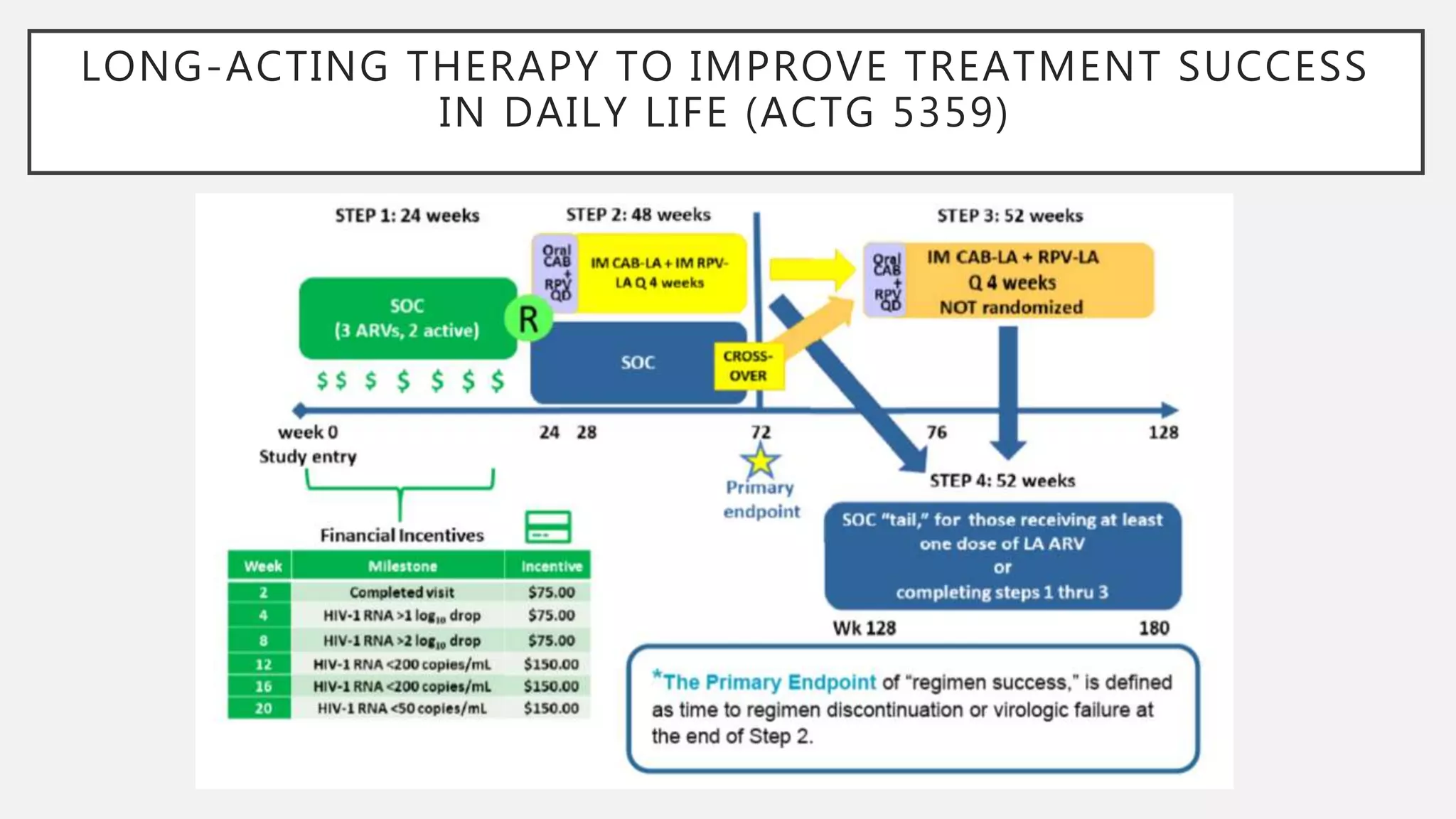
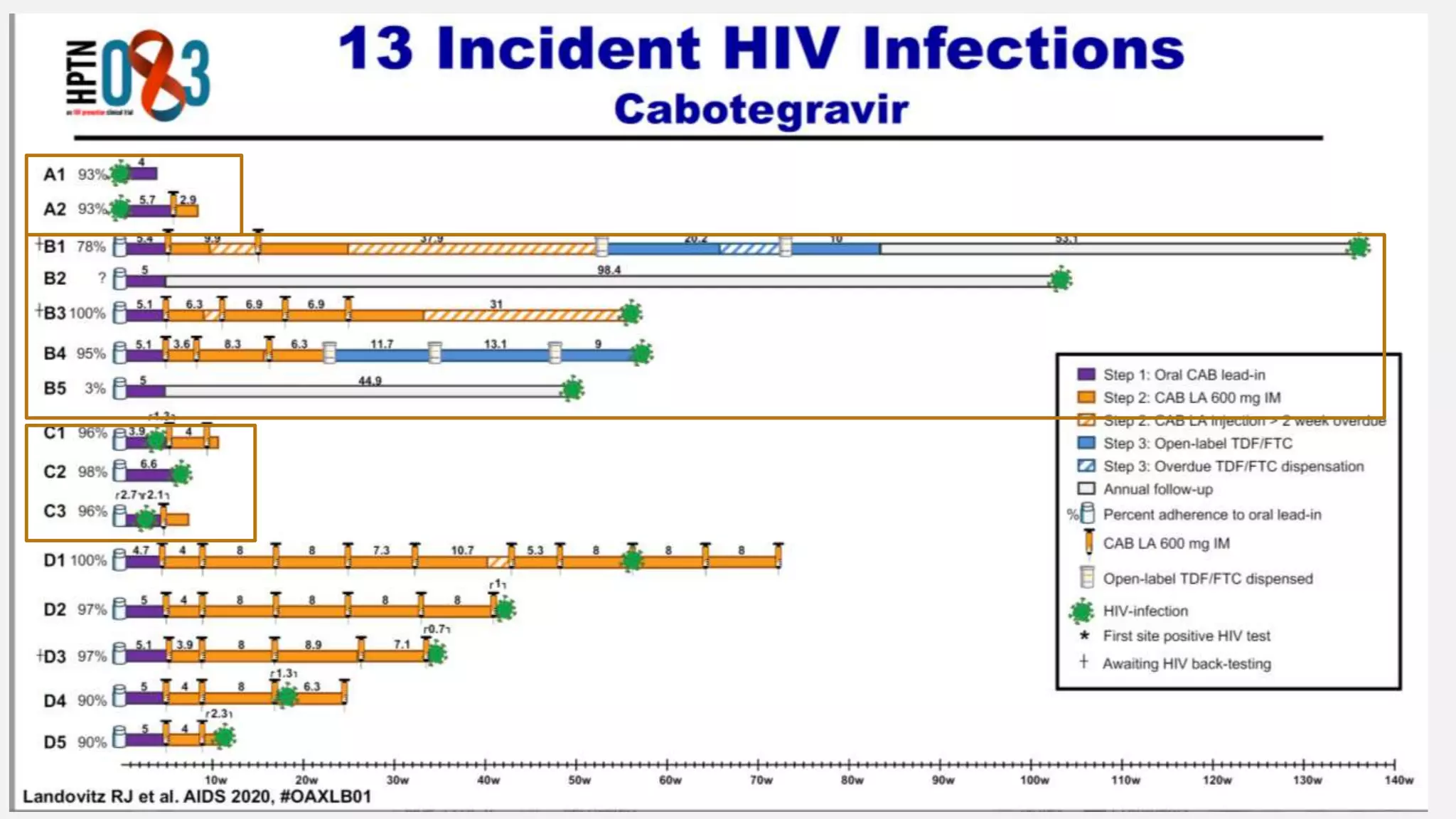
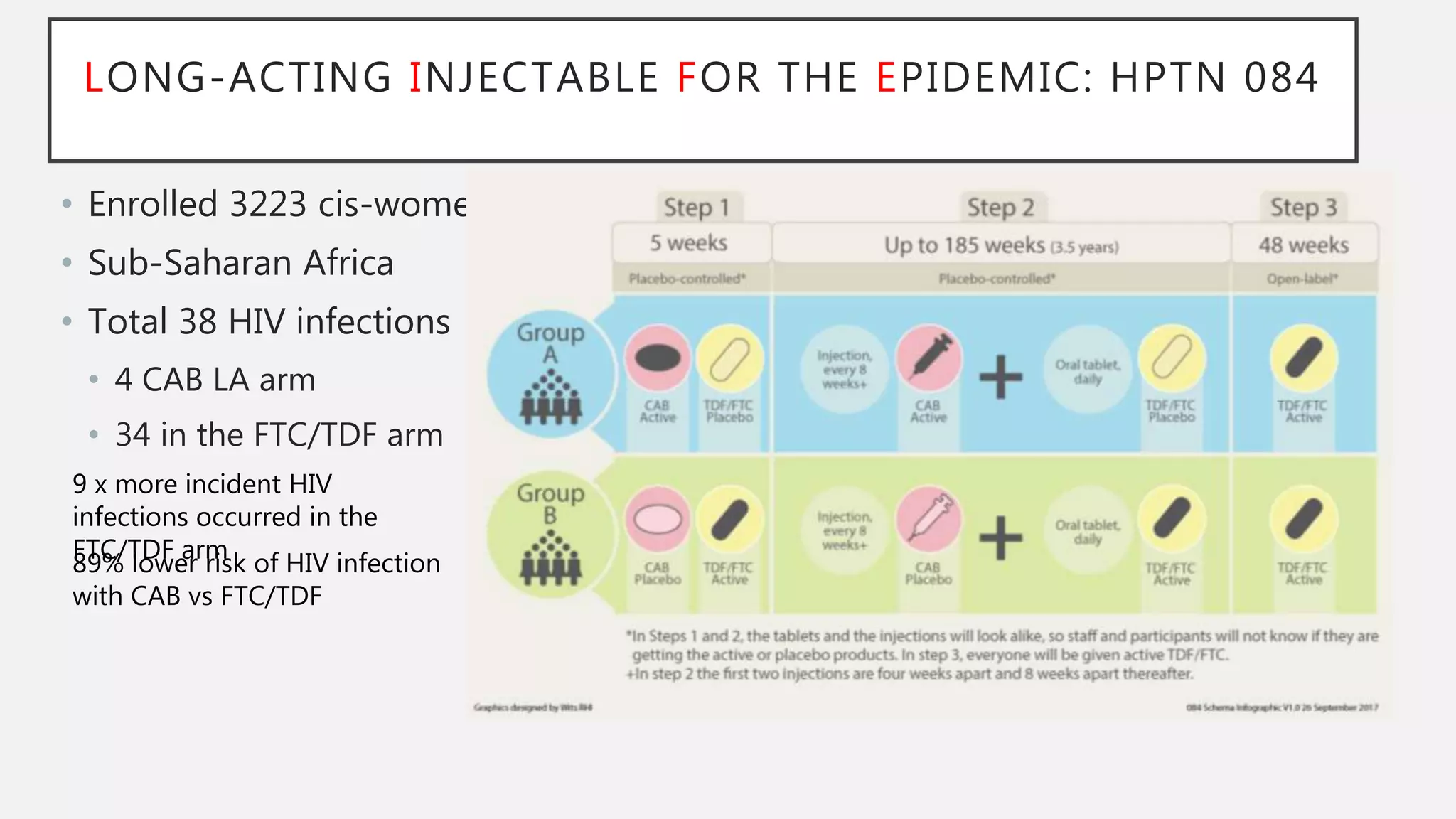
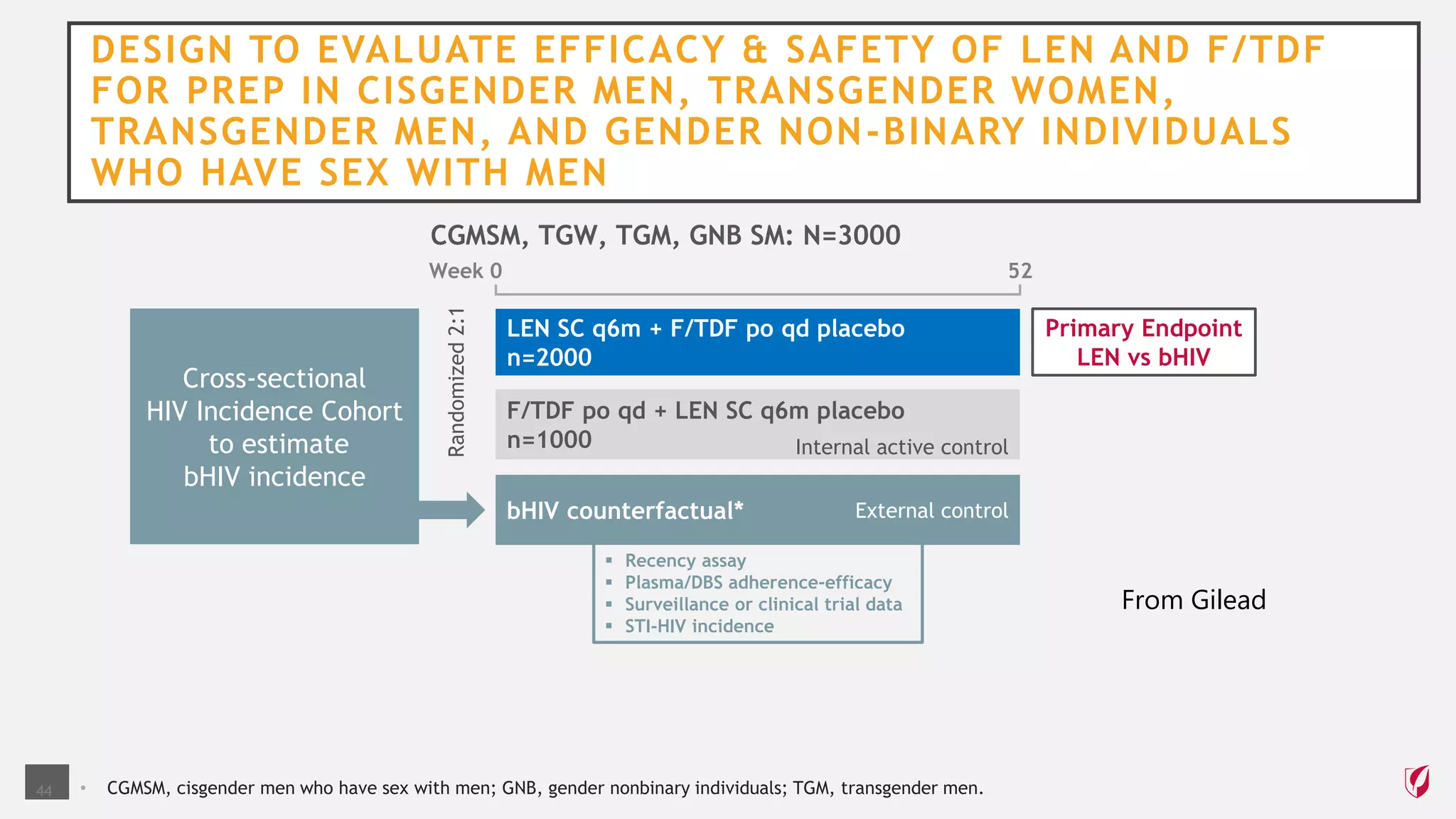
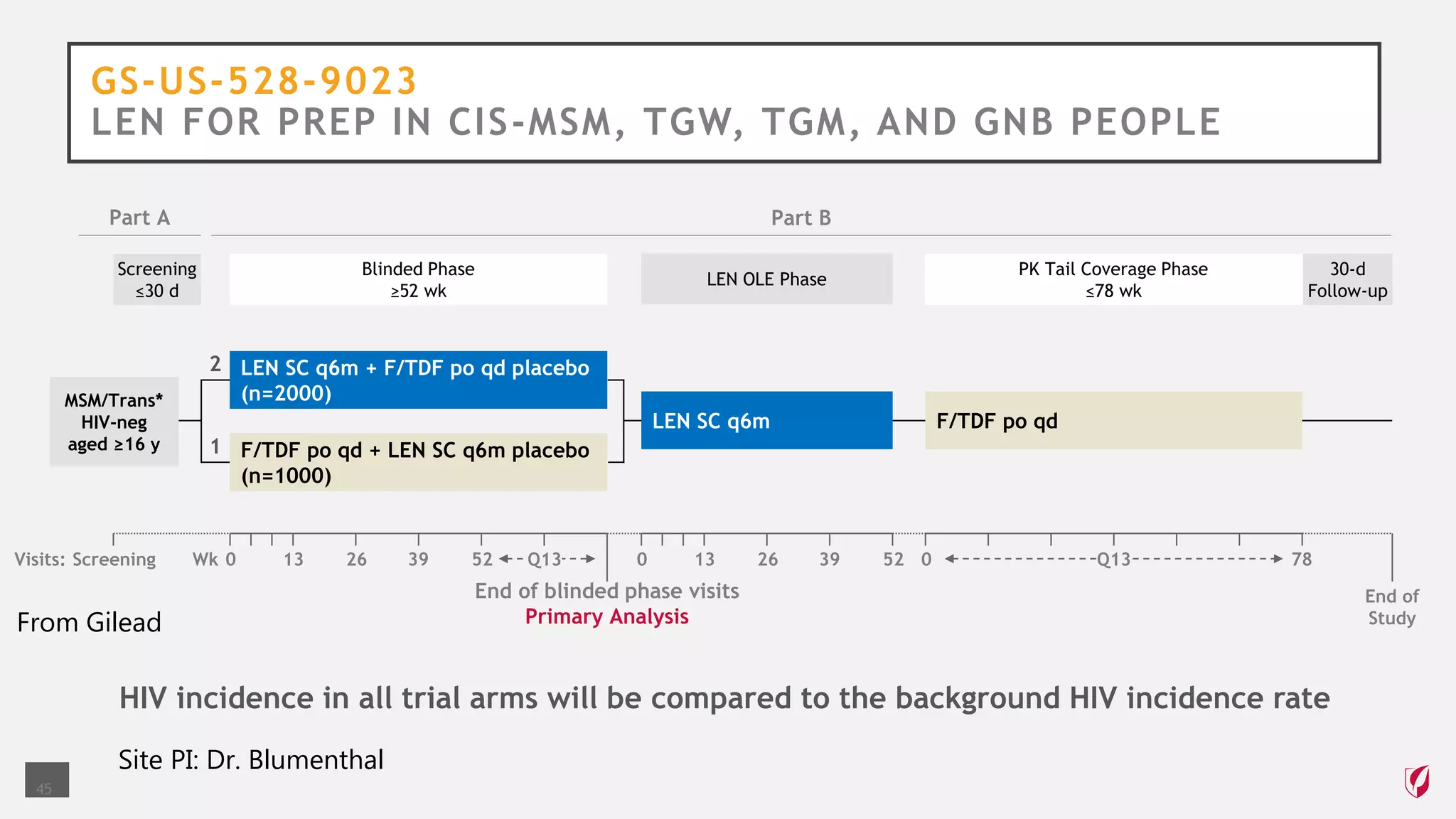
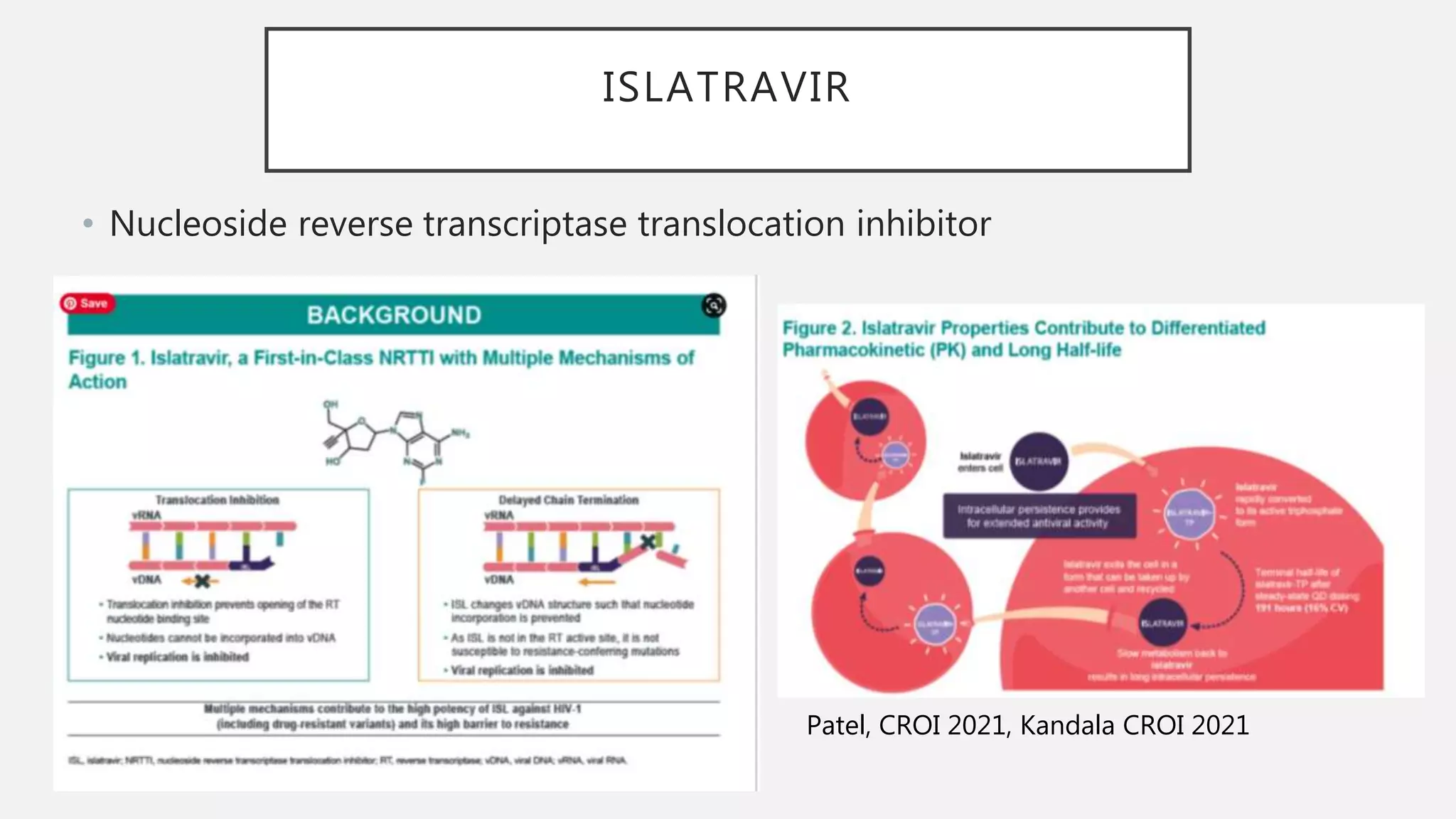
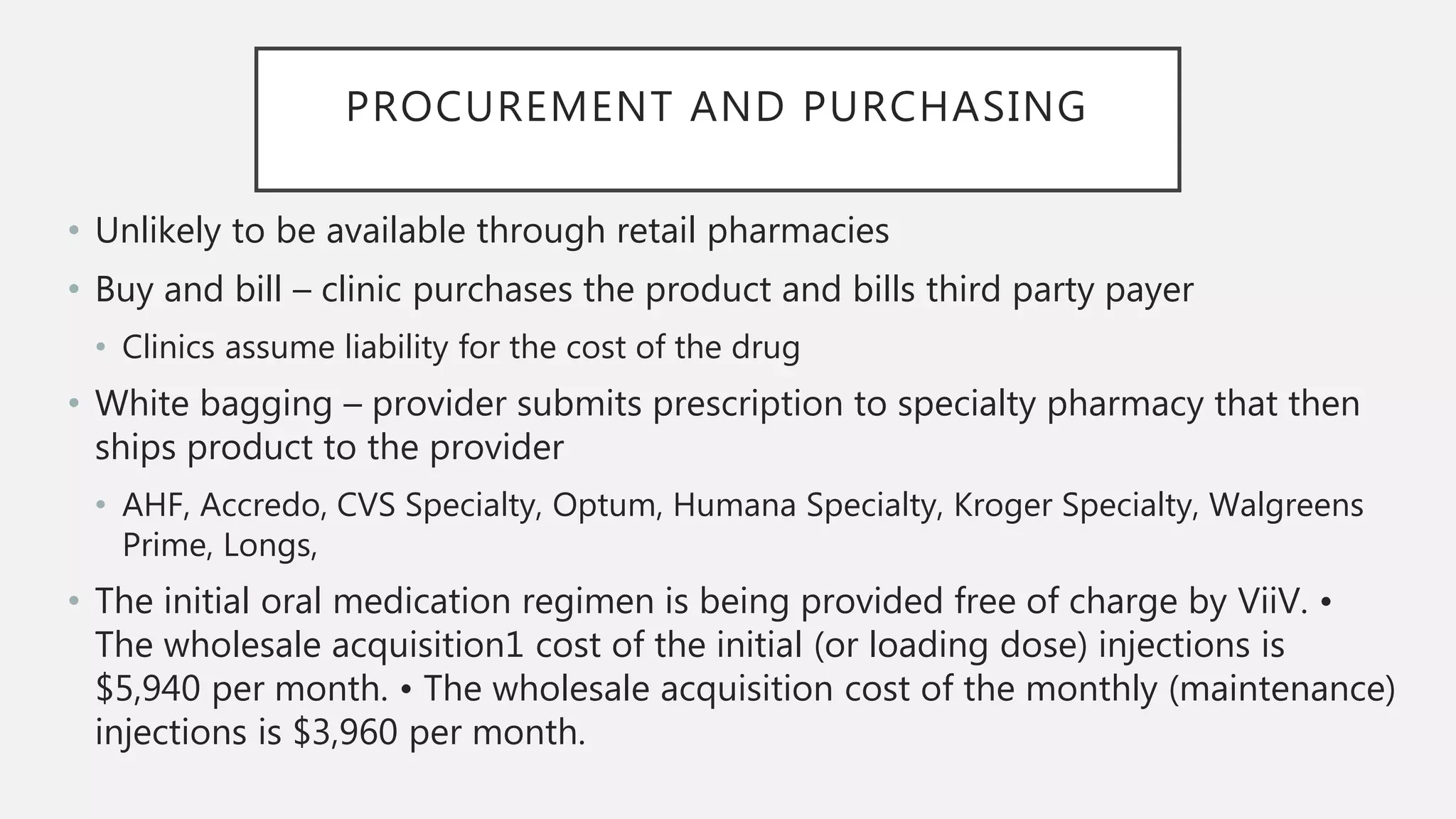
The UC San Diego Antiviral Research Center conducts weekly presentations on current research and practices regarding HIV and other global infectious diseases. The latest presentation discusses the advantages of long-acting injectables for HIV care, highlighting improved adherence and potential benefits for patients. Key studies are reviewed, including the effectiveness and safety of cabotegravir and rilpivirine in various treatment settings.