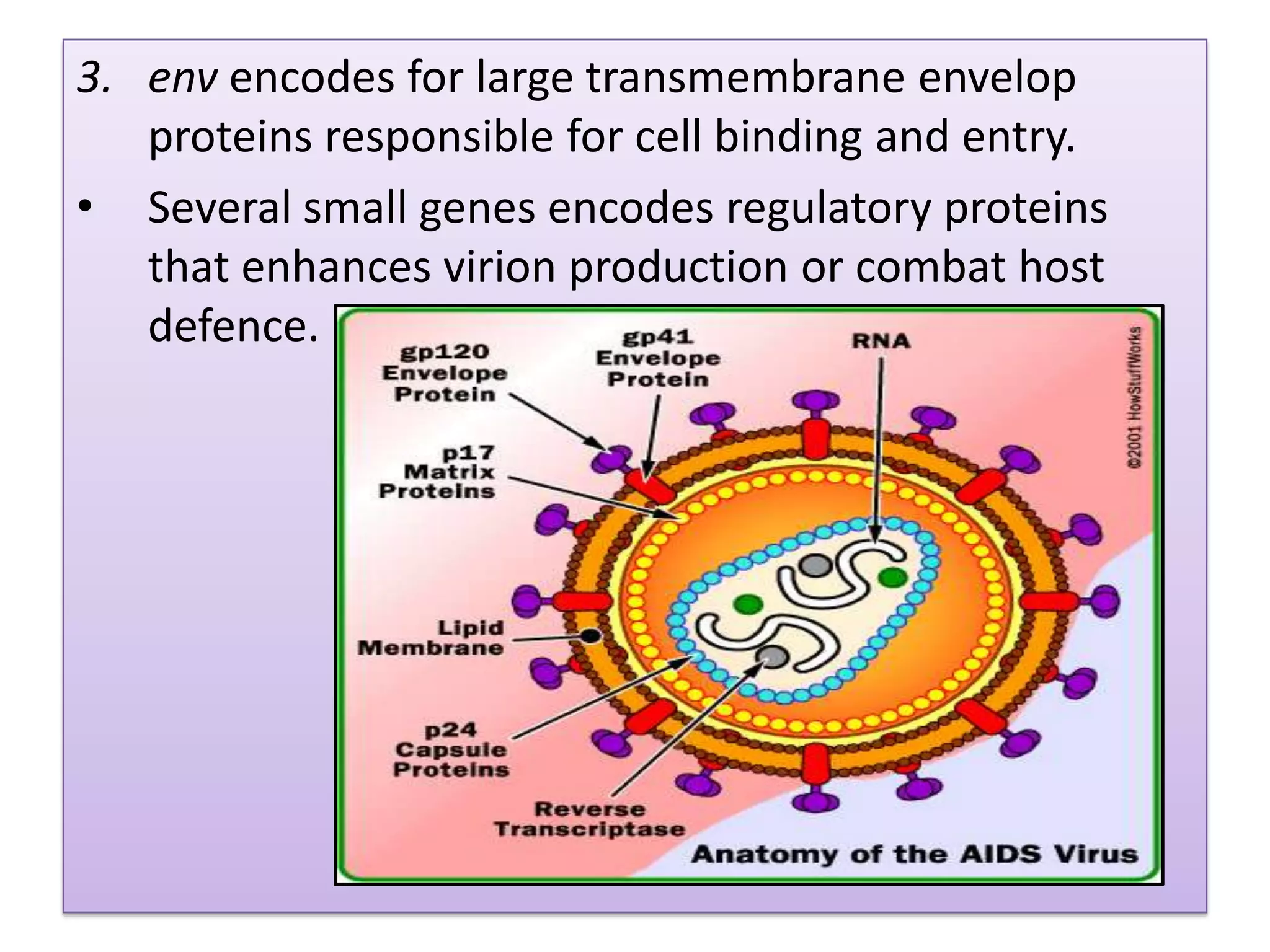
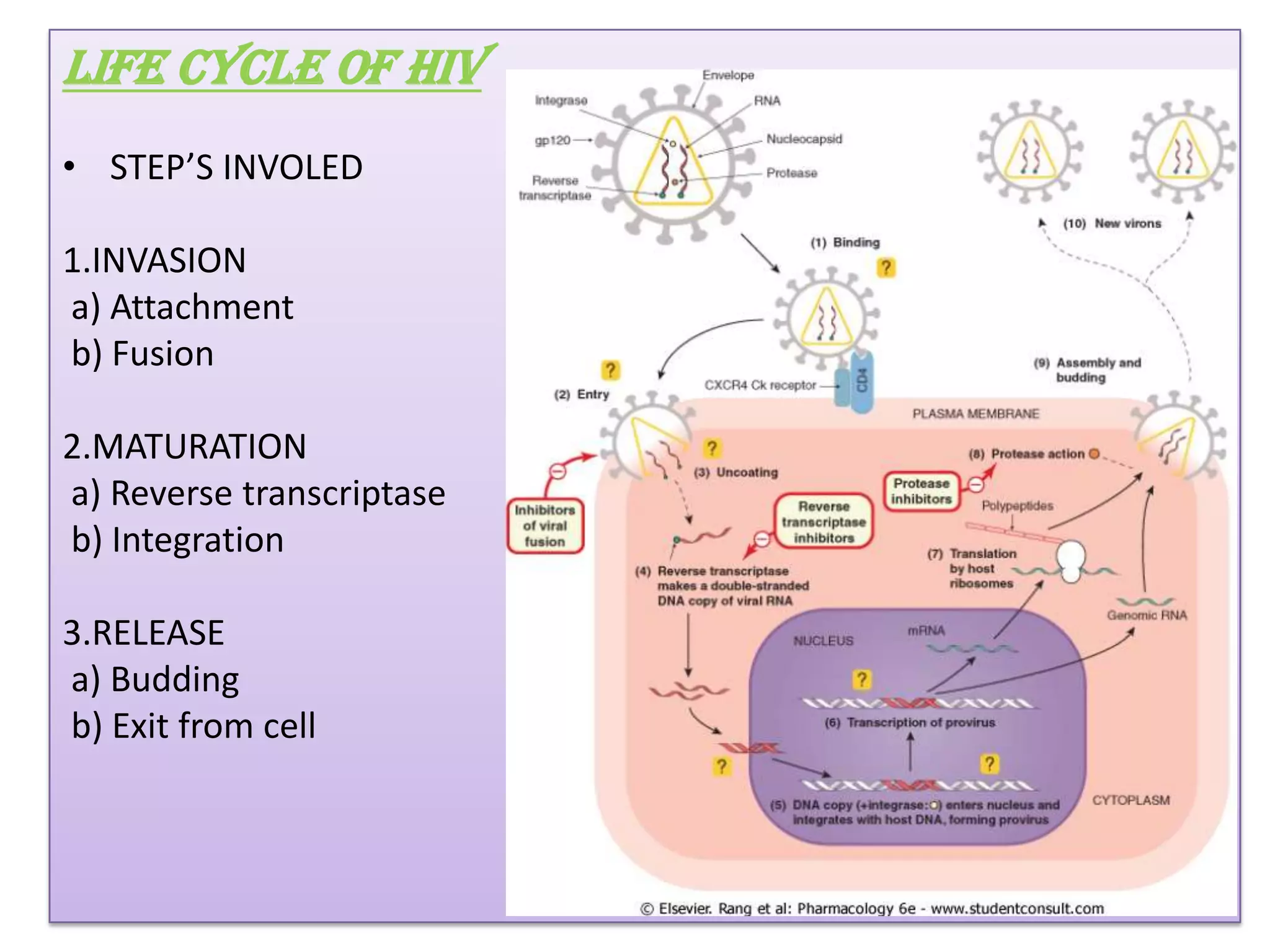
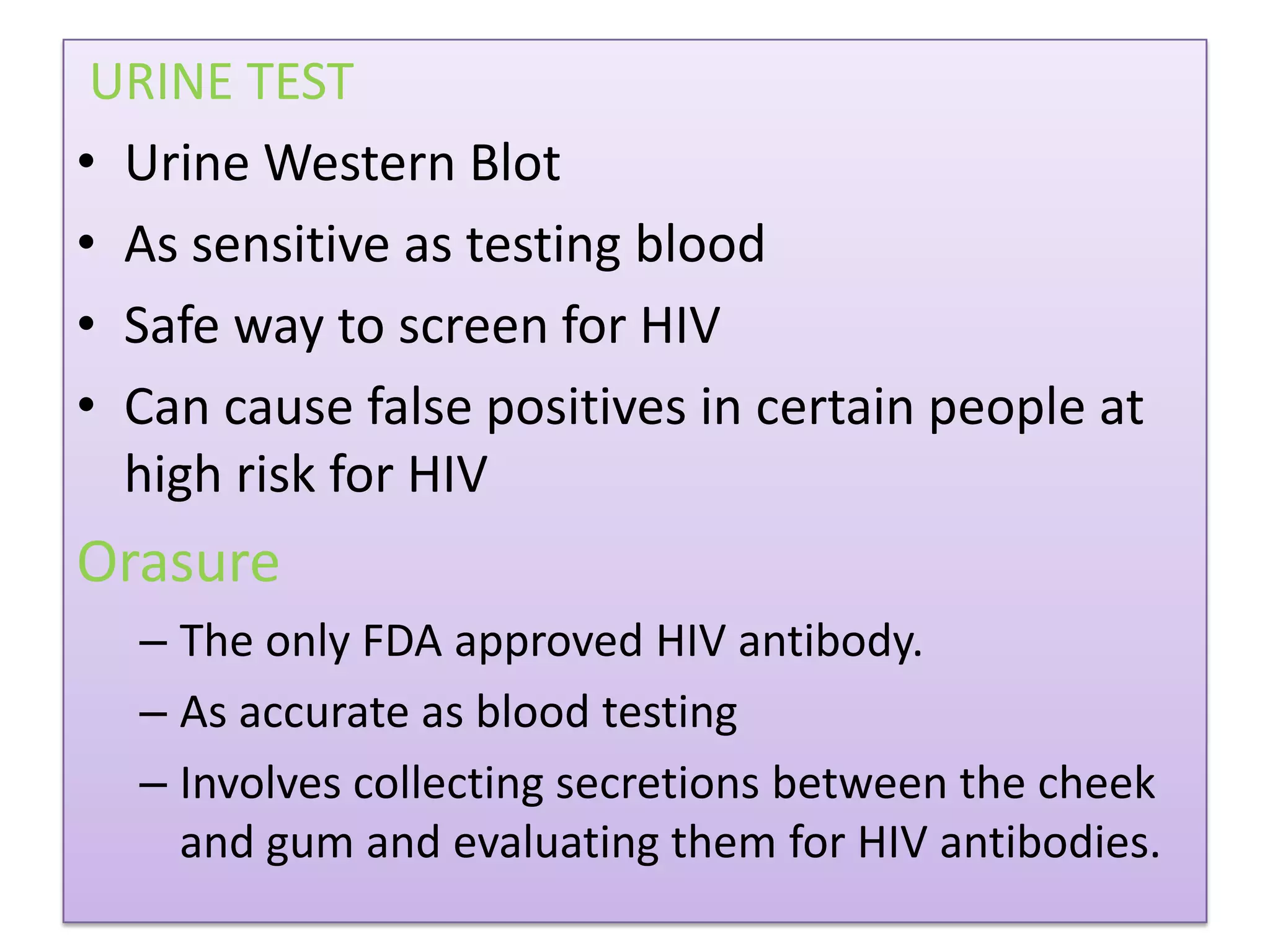
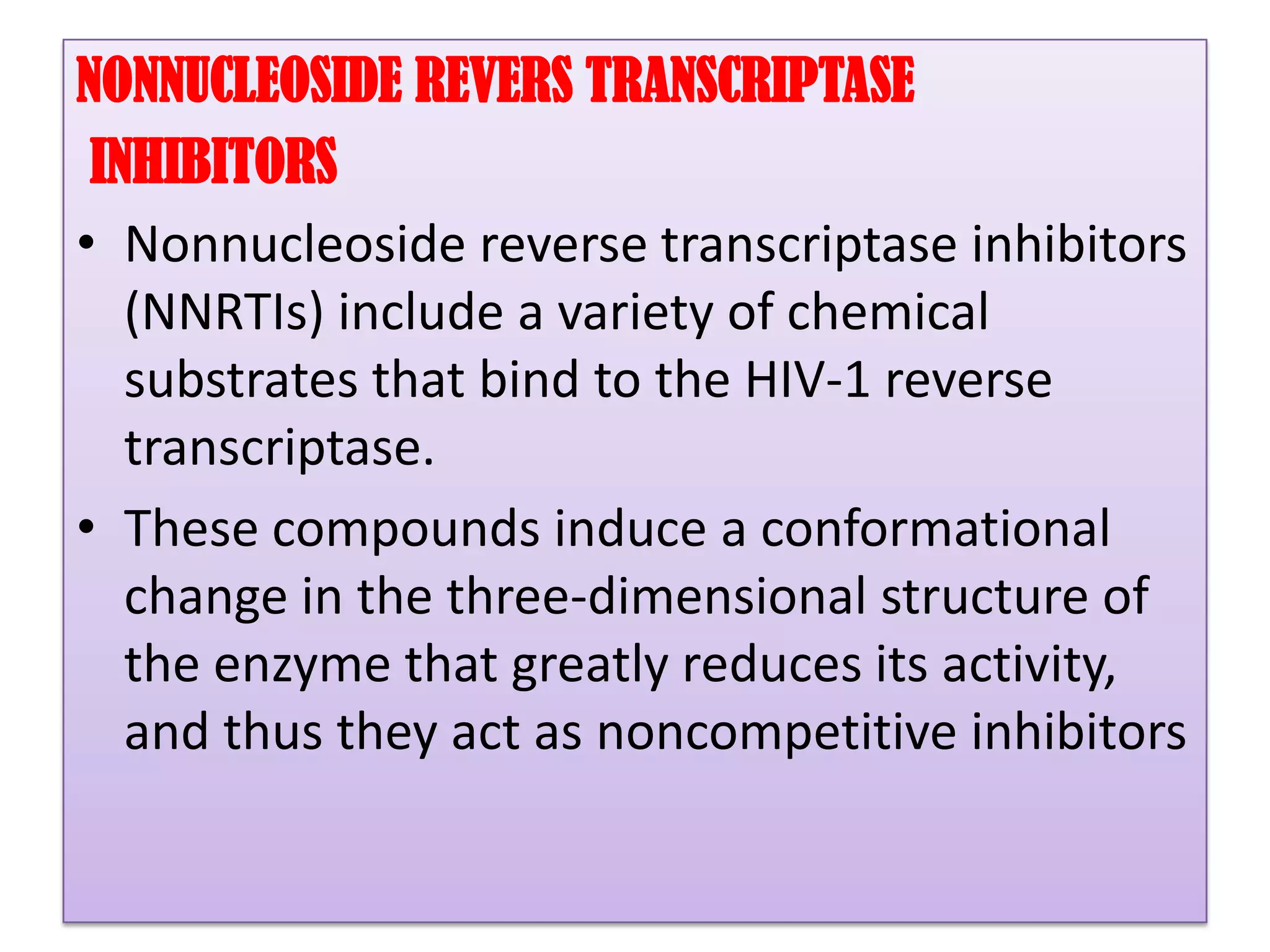
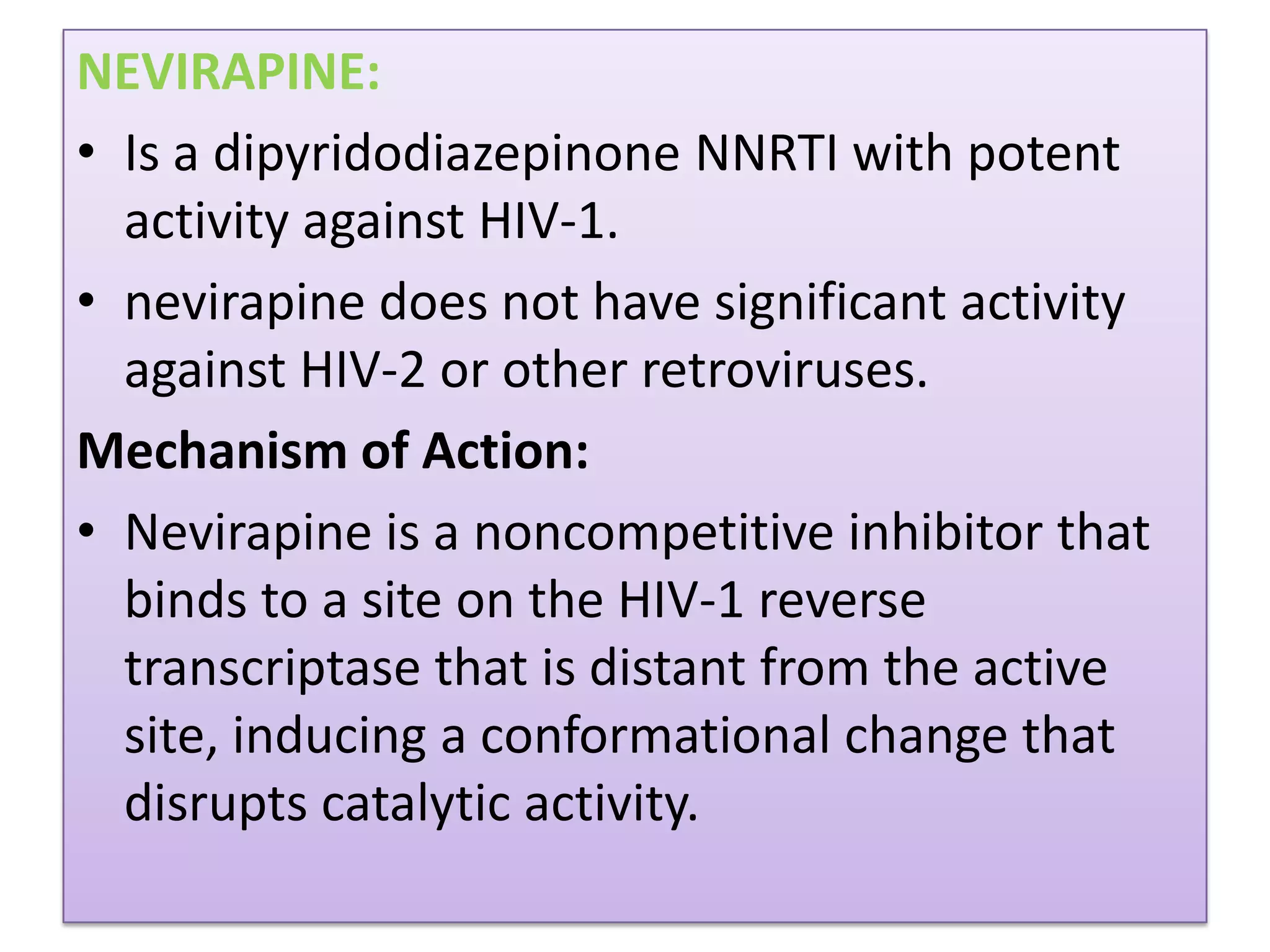
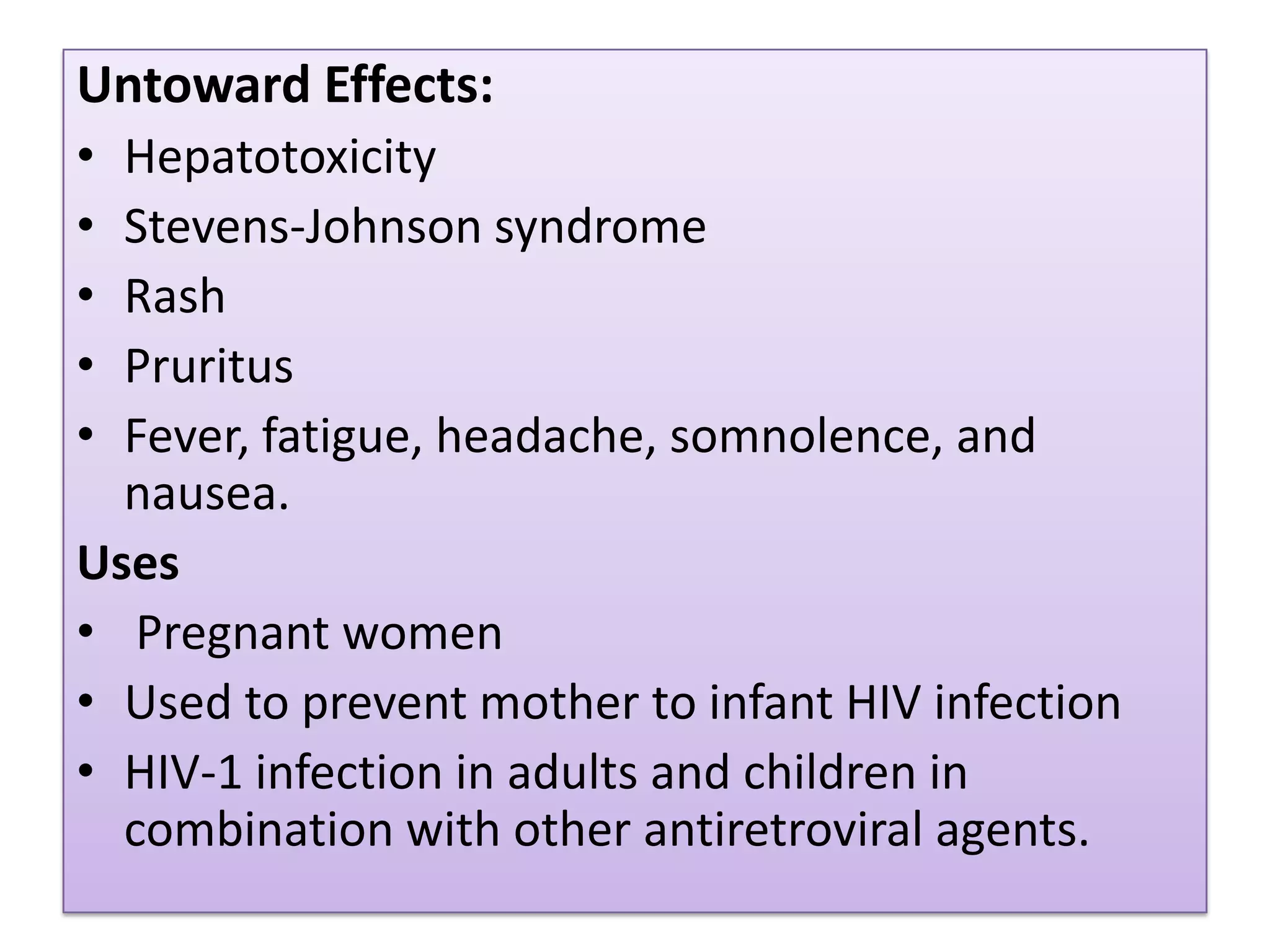
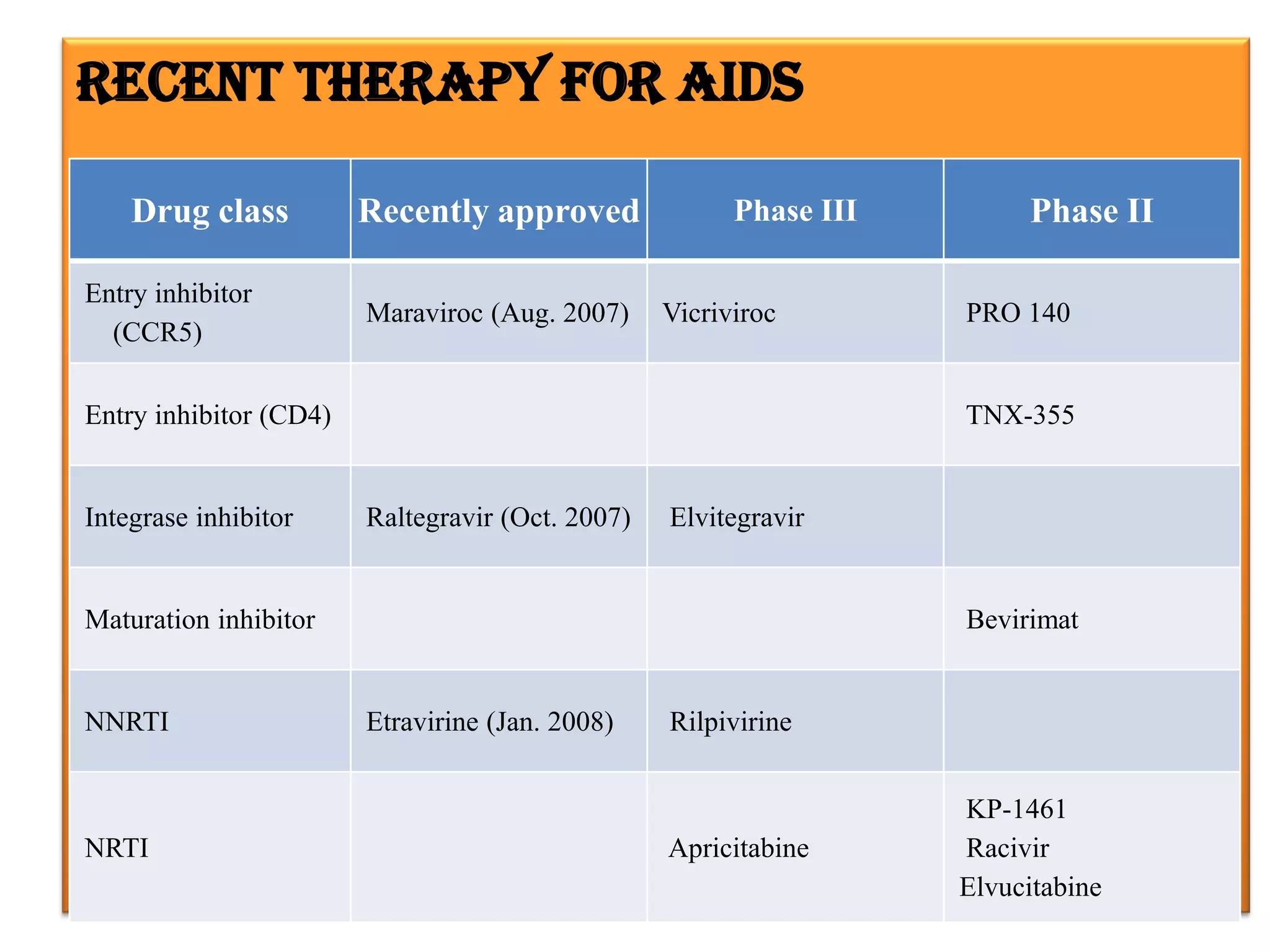
This document provides an overview of AIDS/HIV, including its etiology, pathogenesis, diagnosis, and treatment. It is caused by the HIV virus, which destroys CD4+ T cells. It discusses the life cycle and structure of HIV, how it is transmitted, and the role of key enzymes like reverse transcriptase. Diagnosis involves blood or urine tests to detect HIV antibodies. Treatment involves antiretroviral drugs that target different stages of the viral life cycle, such as reverse transcriptase inhibitors, protease inhibitors, and integrase inhibitors. Vaccine development and other new therapies like gene and monoclonal antibody therapies are also discussed.