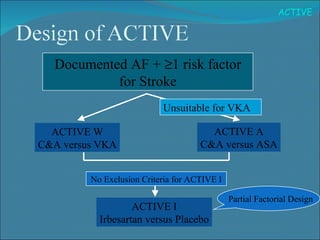
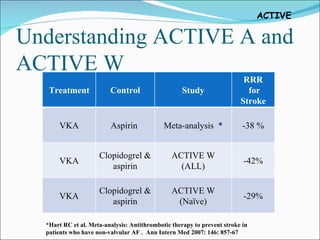
1) The study tested adding clopidogrel to aspirin in patients with atrial fibrillation who were unsuitable for vitamin K antagonist therapy.
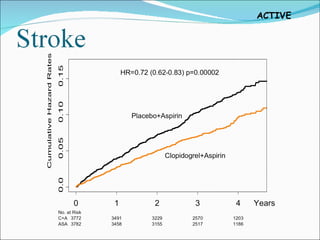
2) It found that adding clopidogrel reduced the risk of major vascular events like stroke or heart attack compared to aspirin alone, with an acceptable increased risk of major bleeding.
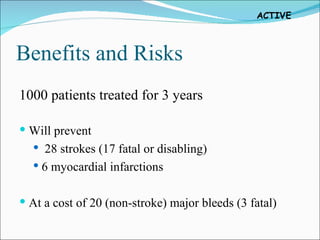
3) For every 1,000 patients treated for 3 years with clopidogrel plus aspirin, it would prevent 28 strokes and 6 heart attacks but result in 20 additional major bleeds, 3 of which would be fatal.