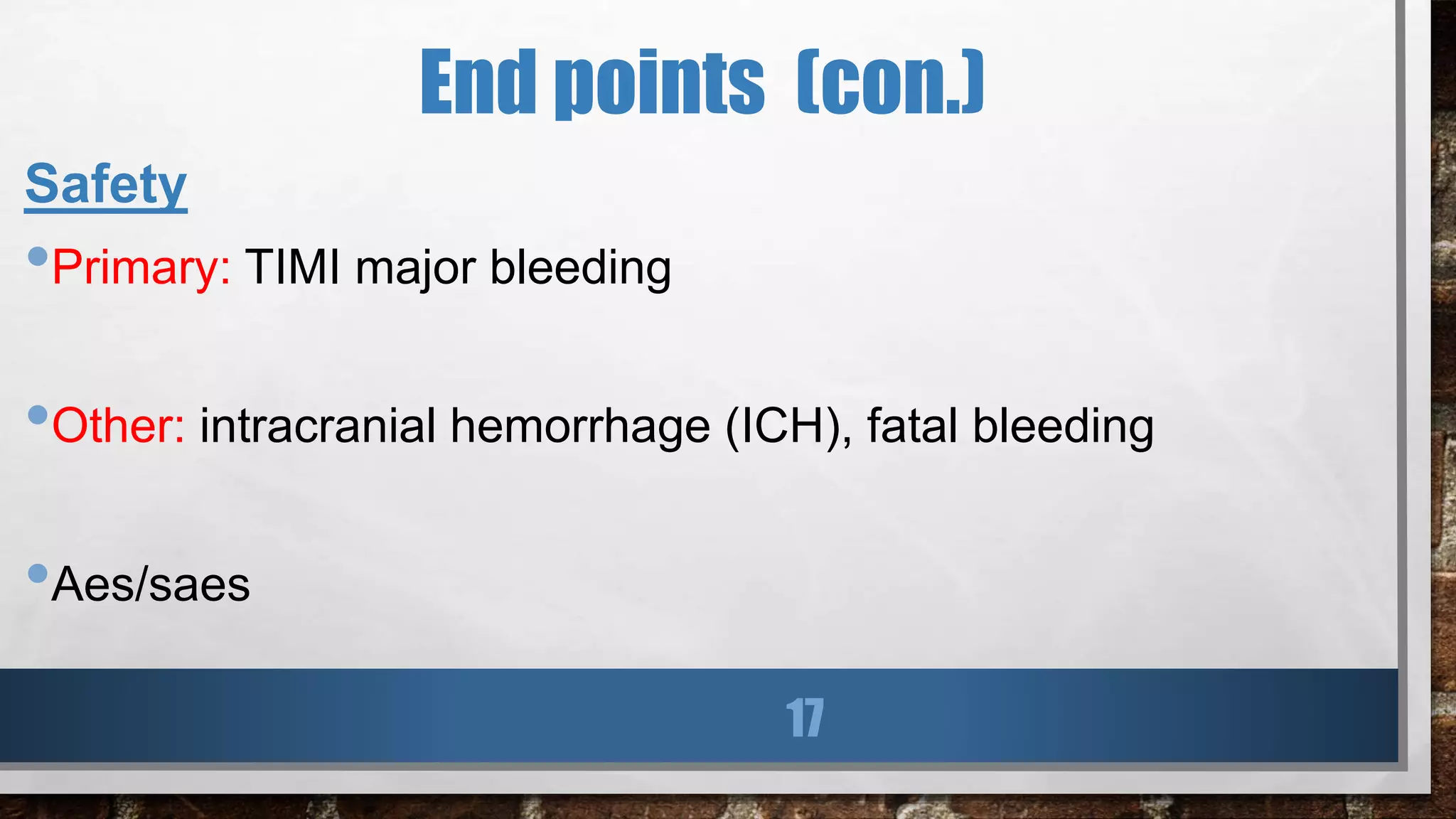
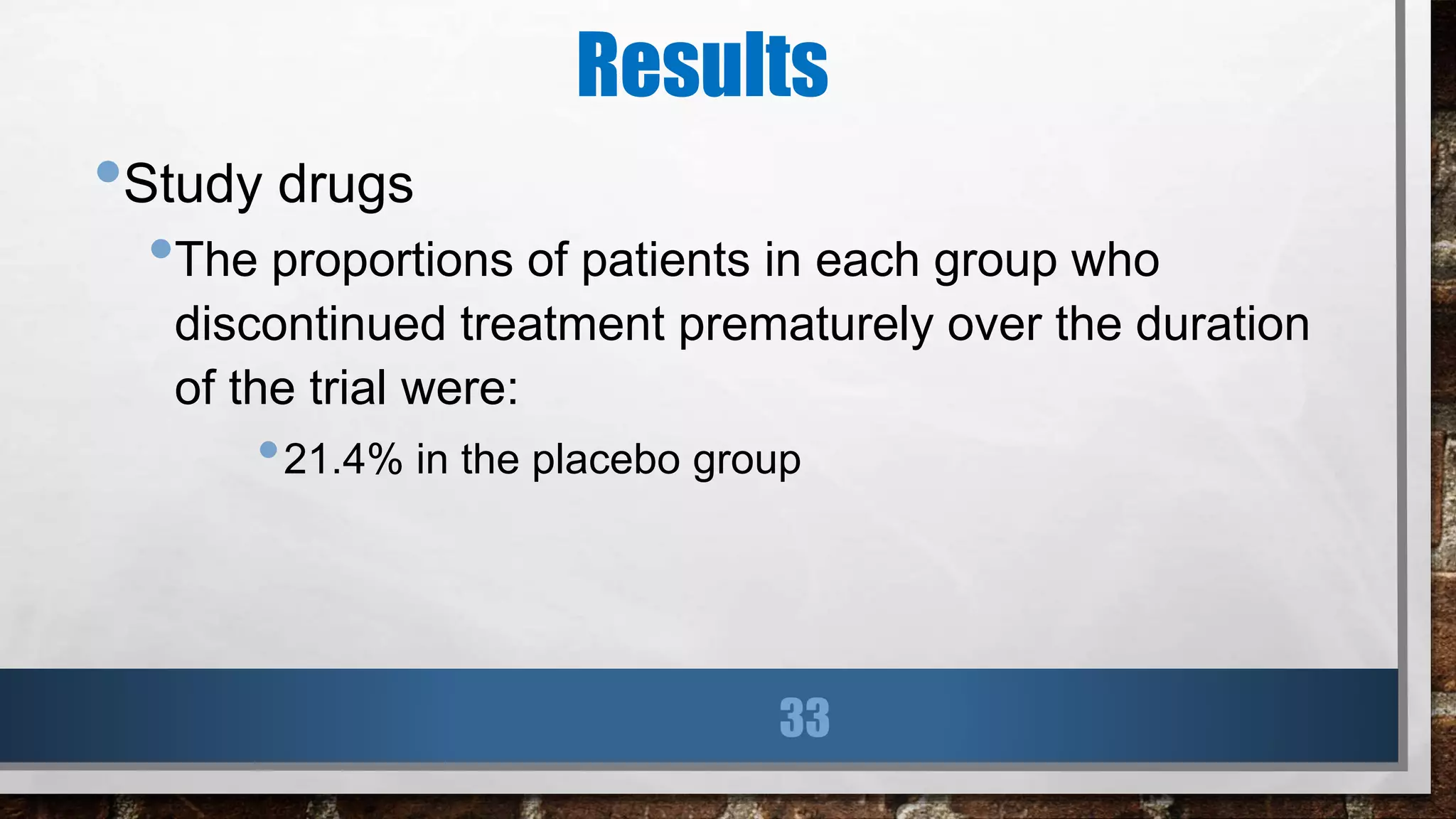
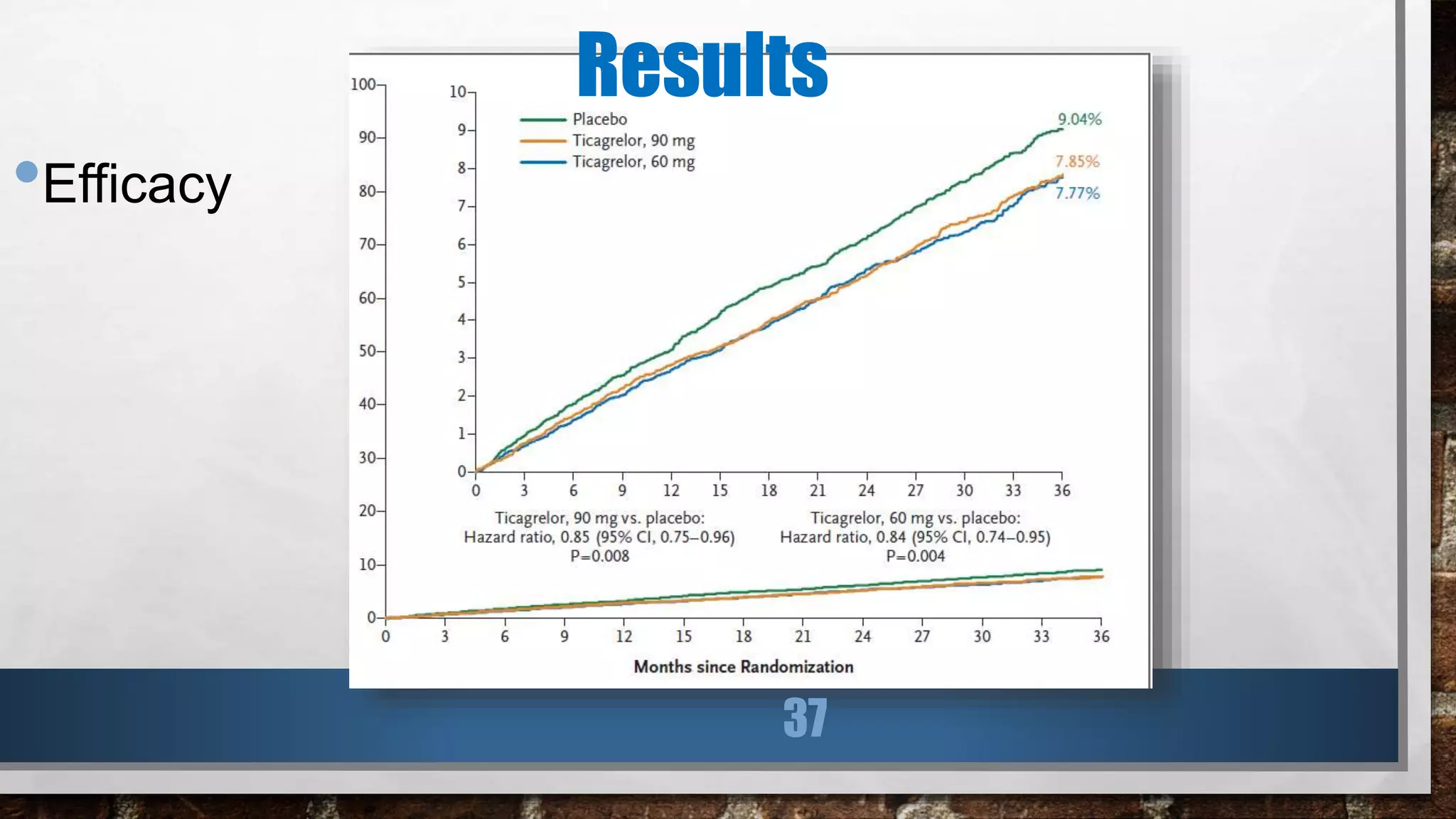
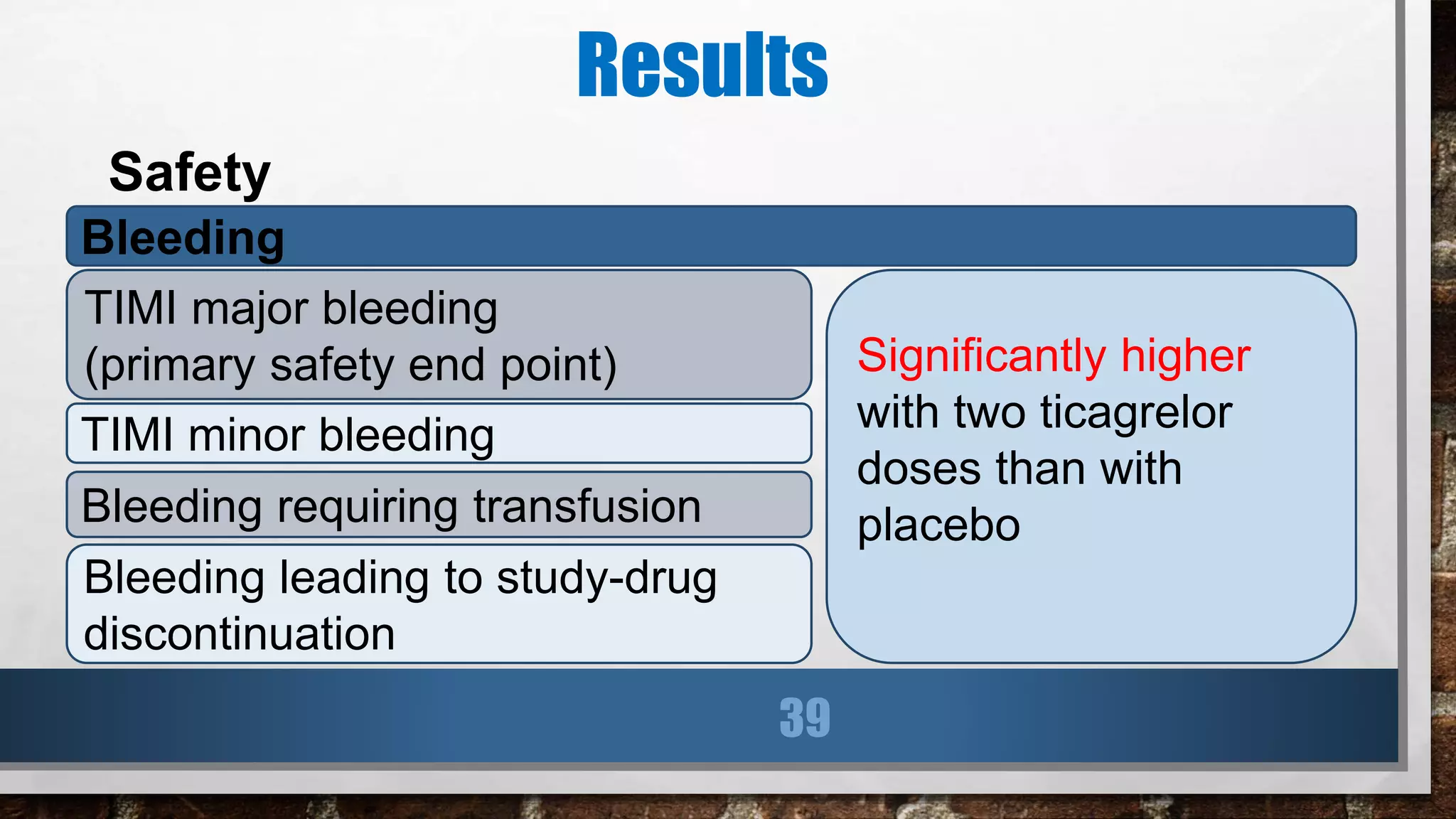
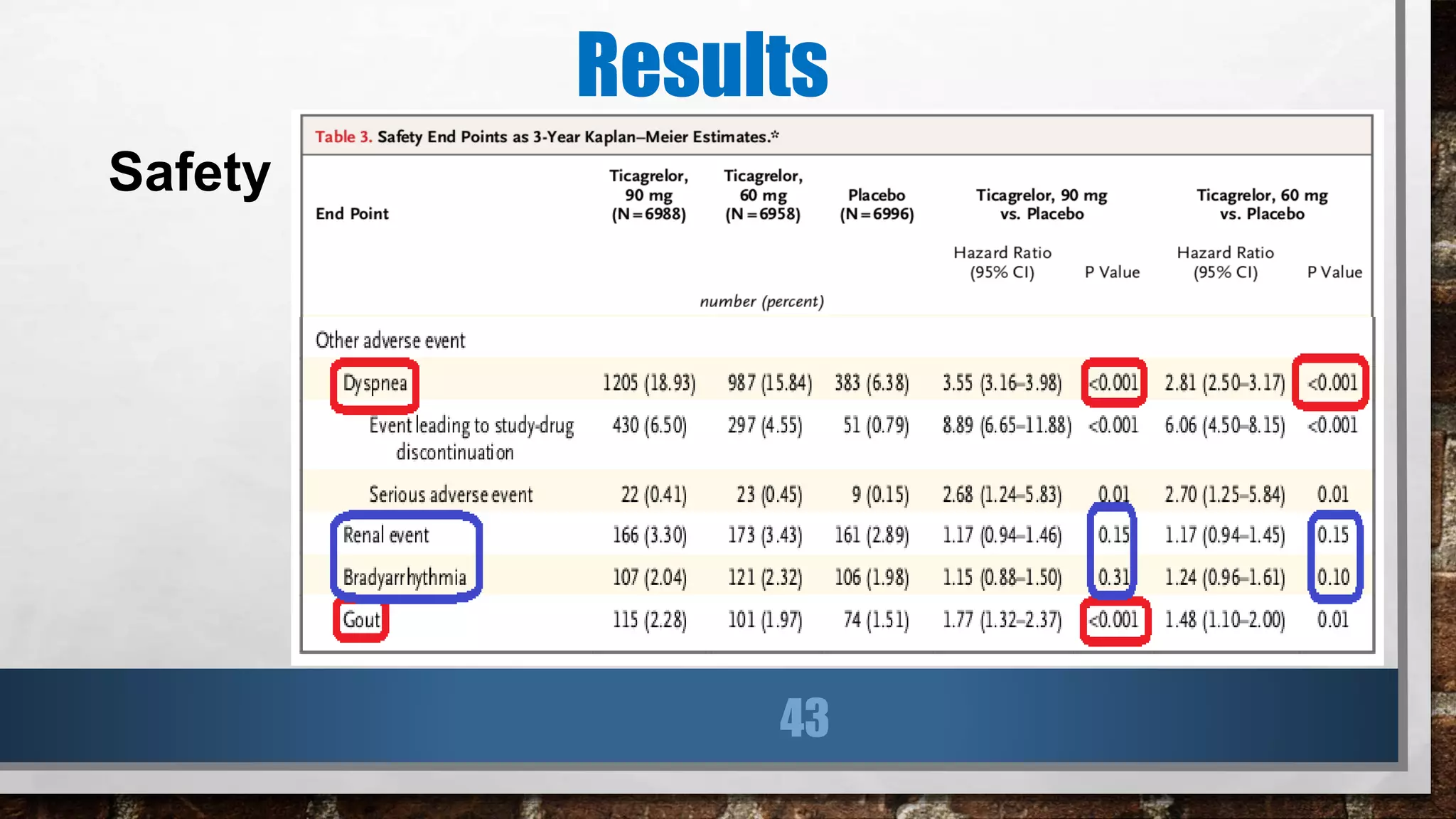
This study evaluated the addition of ticagrelor at doses of 90 mg or 60 mg twice daily to low-dose aspirin in reducing cardiovascular events in patients with a history of myocardial infarction 1 to 3 years prior. Over a median follow-up of 33 months, both ticagrelor doses significantly reduced the primary composite outcome of cardiovascular death, myocardial infarction, or stroke compared to placebo. However, both ticagrelor doses increased the risk of TIMI major bleeding compared to placebo. The 60 mg dose resulted in lower rates of bleeding and dyspnea with similar efficacy compared to the 90 mg dose.