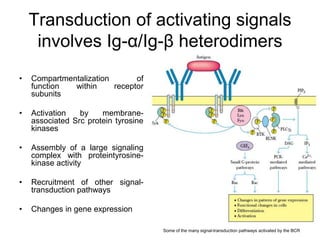
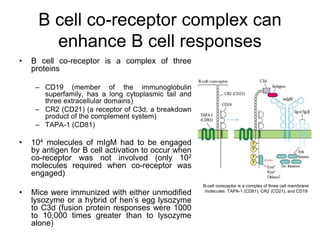
The document summarizes B cell development, activation, and differentiation. It is divided into three main stages: 1) generation of mature B cells, 2) activation of mature B cells, and 3) differentiation into plasma and memory B cells. The process involves an antigen-independent maturation phase and an antigen-dependent activation and differentiation phase where B cells proliferate, undergo affinity maturation and class switching in germinal centers. Cytokines also play a role in B cell differentiation.