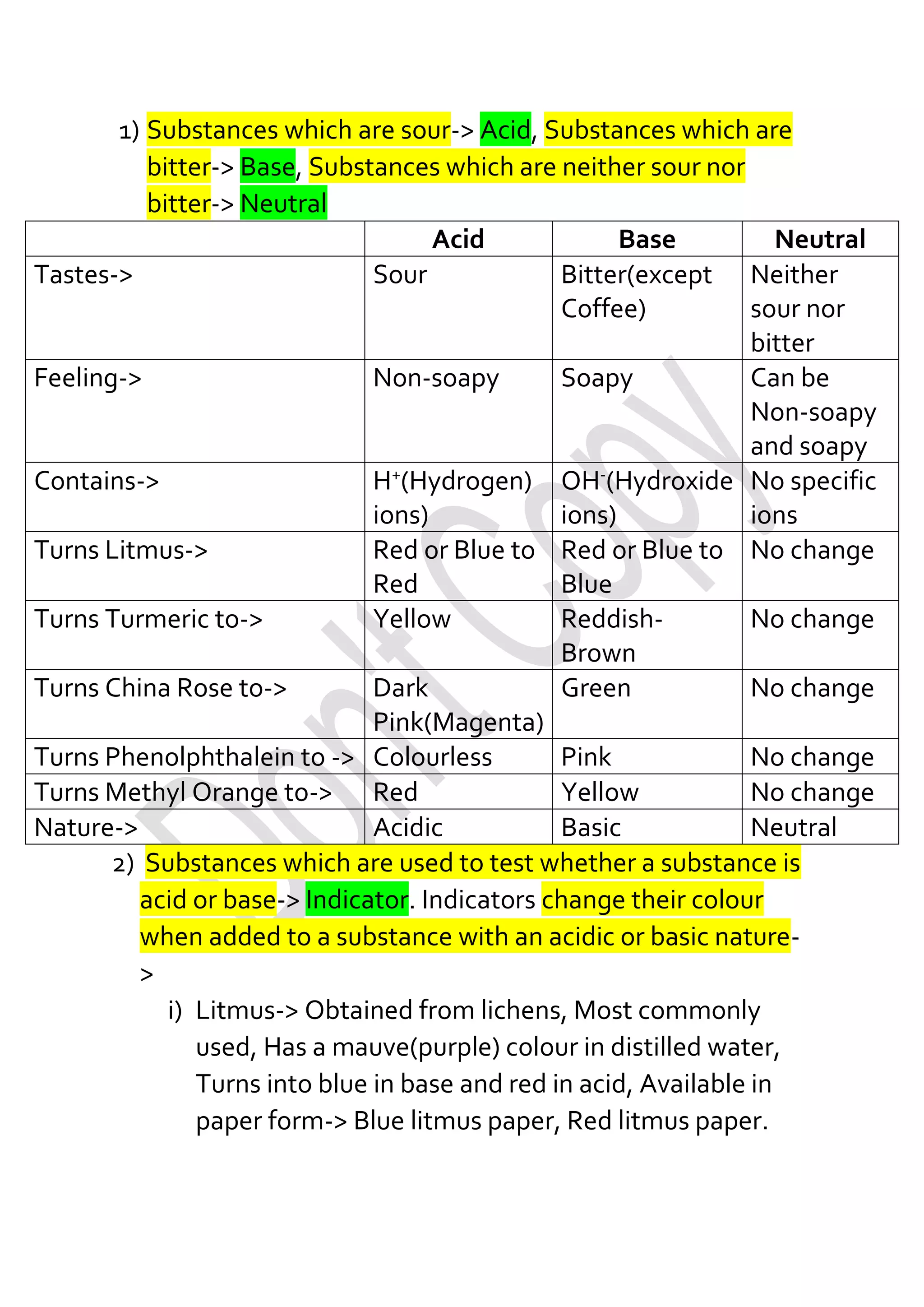
The document explains the classification of substances as acids, bases, or neutrals, along with indicators used to test these properties. It covers topics such as acid rain, neutralization reactions, and practical applications of acids and bases in daily life, including indigestion and soil treatment. Additionally, it lists common acids and bases with examples of their sources.