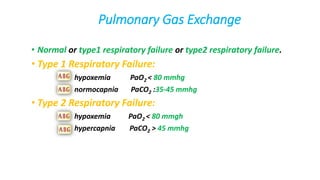
The document provides a comprehensive overview of arterial blood gas (ABG) analysis, detailing its importance in assessing a patient's oxygenation and acid-base balance, along with the steps for interpretation and common acid-base disorders. It explains normal values for various ABG components, the significance of different respiratory failures, and the classification of acid-base disorders based on pH, PaCO2, and HCO3 levels. Additionally, it covers complications, contraindications, and examples of ABG interpretations in clinical practice.








![Components Of ABG?
❑ PH [H+]
❑ PaCO2 partial pressure CO2
❑ PaO2 partial pressure O2
❑ HCO3 Bicarbonate
❑ SaO2 Oxygen saturation
❑ BE Base excess
❑ Others….](https://image.slidesharecdn.com/abg2-220428134619/85/ABG-pptx-9-320.jpg)























![Anion Gap
• If the ABG results in (metabolic acidosis) then we
should measure anion gap.
• Normally: AG= 12±4.
• Anion gap(AG) = Na+ - [Cl- + HCO3
-]
• Na: 150 mmol/L , HCO3: 15 , Cl:100
So 150-(100+15)
150-115= 35 its High](https://image.slidesharecdn.com/abg2-220428134619/85/ABG-pptx-35-320.jpg)


