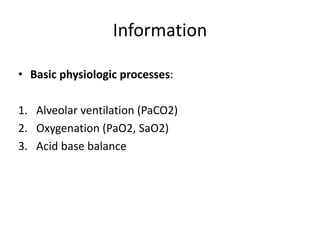
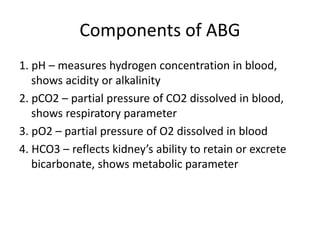
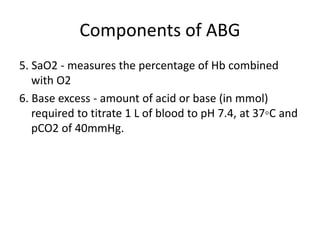
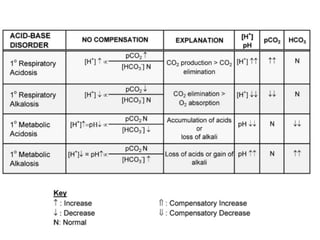
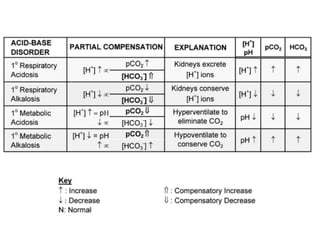
An arterial blood gas (ABG) test measures oxygen and carbon dioxide levels and acidity in arterial blood. It is useful for assessing ventilatory status, oxygenation, and acid-base balance. Blood is drawn from an artery, usually the radial artery. Abnormal ABG values can provide insight into underlying pulmonary or metabolic conditions. The bicarbonate-based Henderson-Hasselbalch approach and Stewart approach can be used to analyze acid-base disorders. Compensation mechanisms aim to return pH to normal levels through respiratory or renal adjustments.










![Stewart approach
• Non bicarbonate based
• Variables :
1. Respiratory – pCO2
2. Metabolic – strong ion difference (SID) and total
weak acids (ATOT)
SID : [cations]-[anions]
ATOT : represent all non bicarbonate buffer](https://image.slidesharecdn.com/abg-210303040944/85/ABG-17-320.jpg)






![Anion gap
• Difference between measured cations and measured
anions :
[Na + K] – [Cl + HCO3]
Normal value : 14-16
Or
[Na] – [Cl + HCO3]
Normal value : 8 -12
• Unmeasured anions : significant proportion e.g –
proteins, phosphates, sulfates, lactates, ketones etc.](https://image.slidesharecdn.com/abg-210303040944/85/ABG-26-320.jpg)








