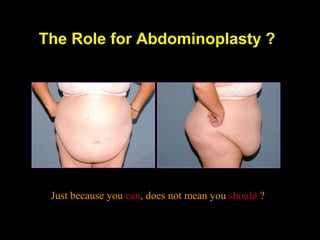
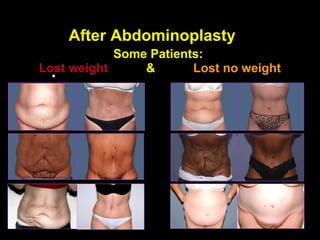
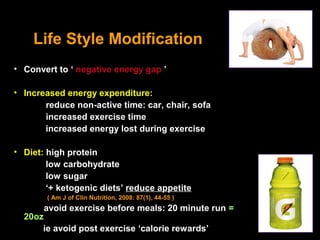
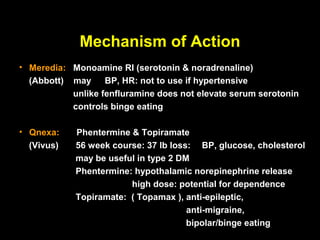
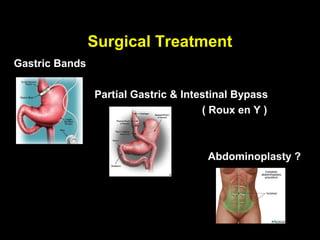
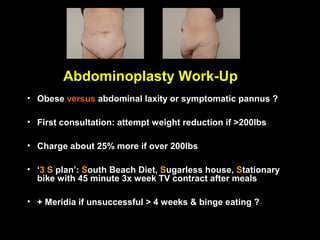
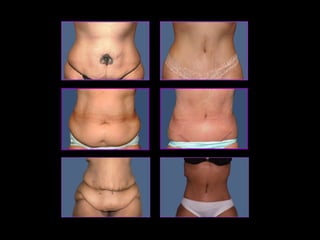
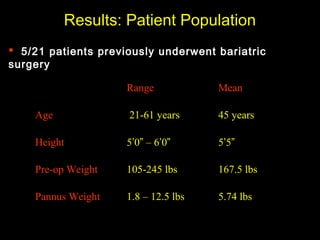
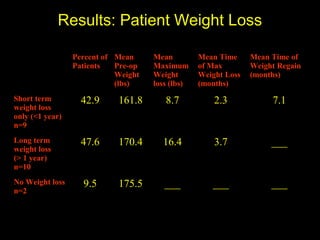
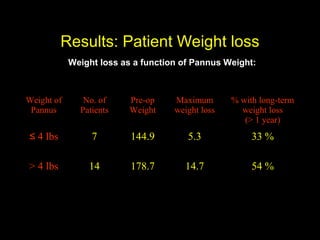
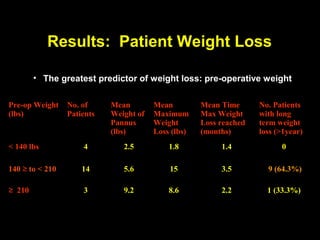
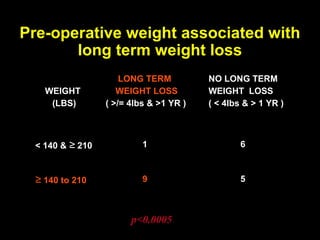
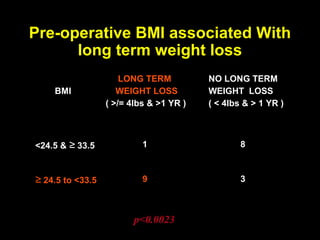
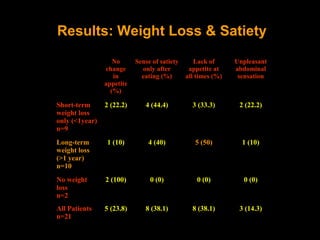
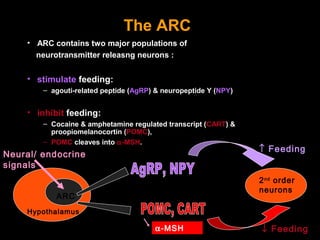
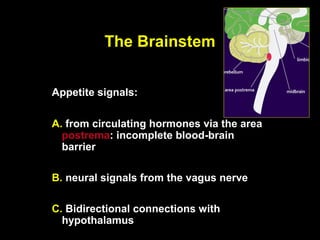
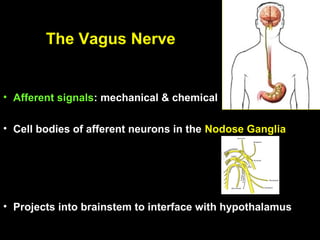
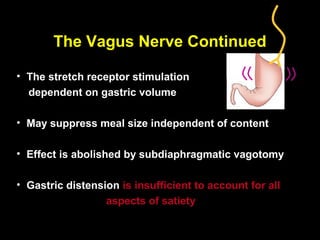
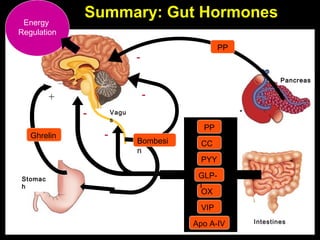
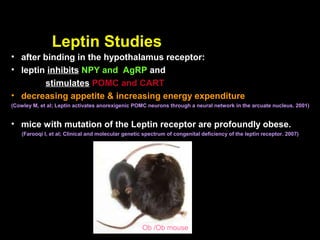
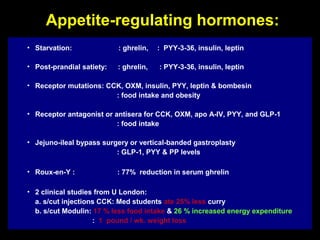
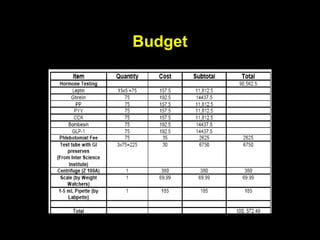
This pilot study investigates the potential for permanent weight reduction following abdominoplasty, focusing on neurocrine factors and patient outcomes. Results indicate that a significant proportion of patients reported weight loss, with increased satiety being a key factor in long-term weight maintenance. The study suggests the importance of pre-operative weight and BMI in predicting weight loss success post-surgery.