Embed presentation






























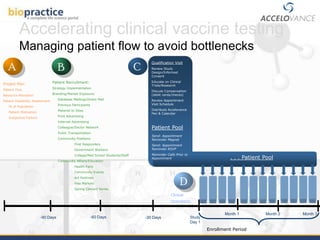
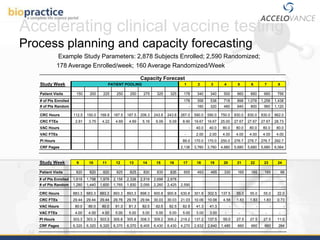

This document provides an overview of clinical vaccine development from a US perspective. It discusses general considerations for vaccine development including safety, efficacy, disease incidence and risk factors. It reviews key FDA guidance documents on topics like Good Clinical Practice standards and the vaccine approval process. It also covers practical clinical development considerations for vaccine trials including safety evaluation, immunogenicity assessment, route of administration, dosing, and the use of boosters. The document is intended as a guide for those developing new vaccines.


































