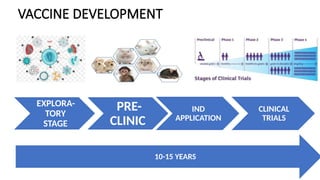
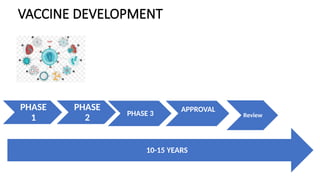
Vaccine development is a lengthy process that spans 10-15 years and includes stages such as exploratory research, preclinical evaluation, and clinical trials before regulatory approval. The process assesses the safety and efficacy of vaccine candidates, starting from laboratory research to human testing, culminating in manufacturing and distribution. Post-marketing surveillance is crucial for monitoring vaccine safety and effectiveness after approval.