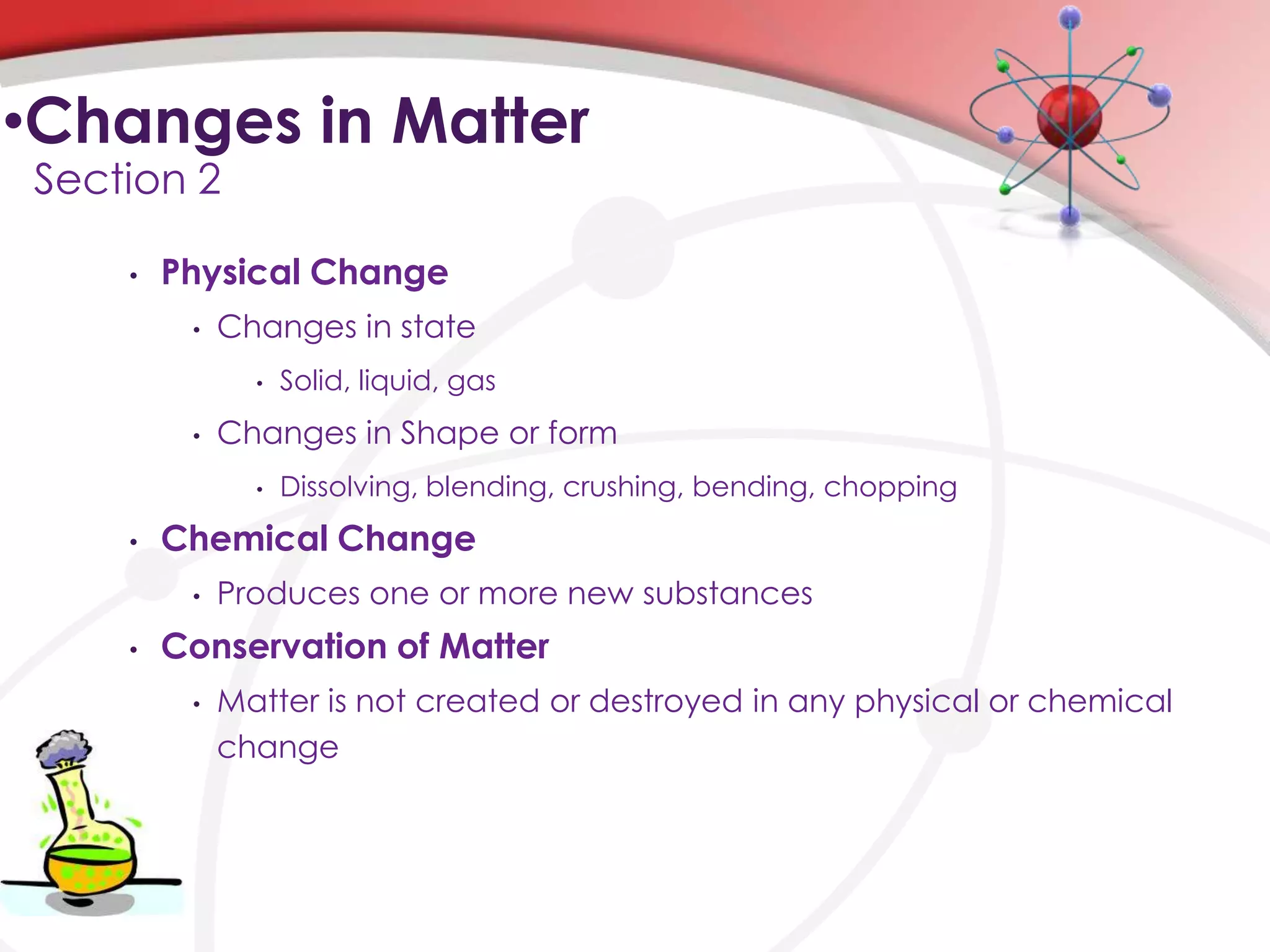
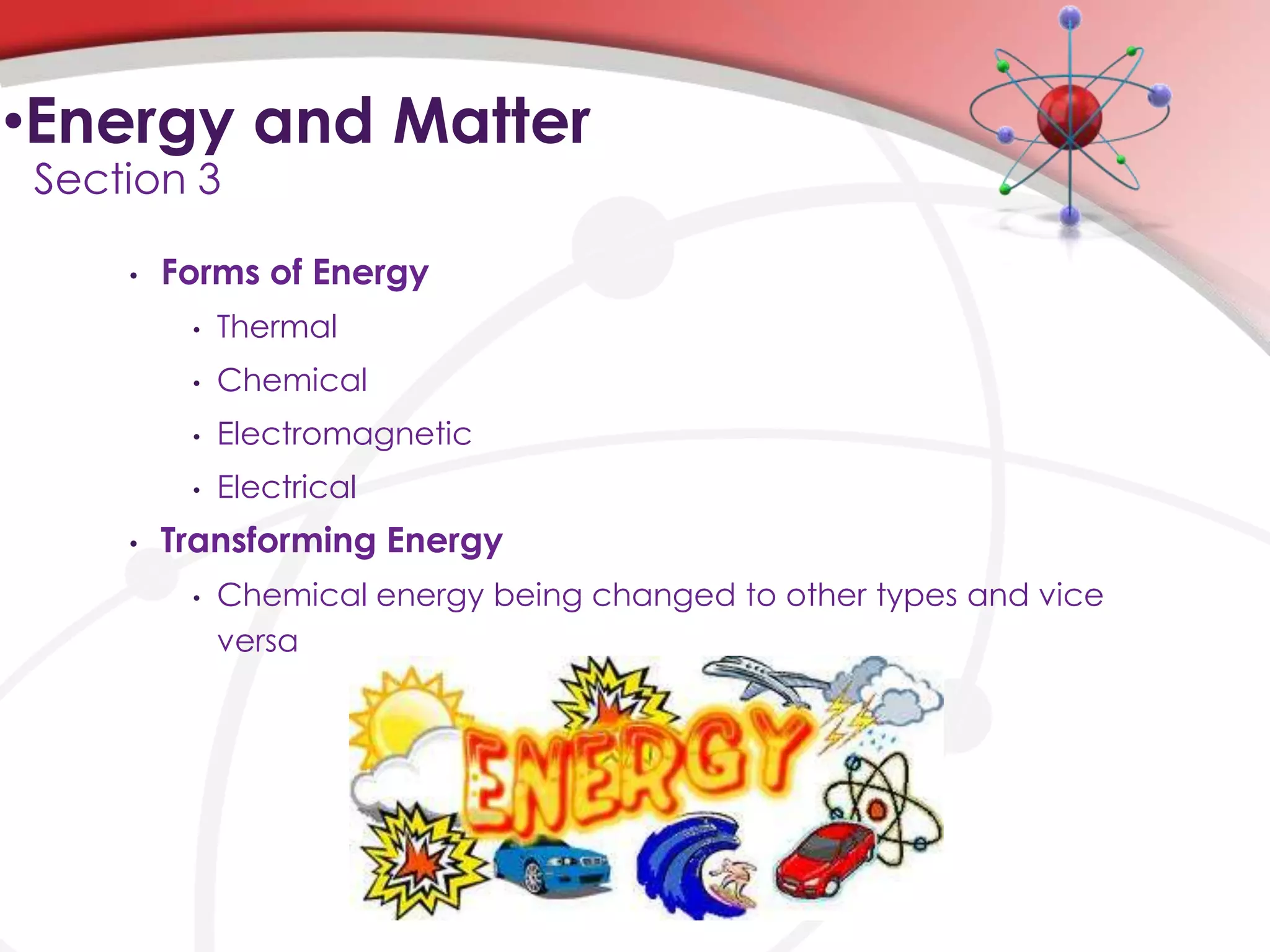
This chapter discusses the nature of matter. It describes matter and the different types including elements, compounds, mixtures, and changes in matter. It also discusses energy and how energy changes occur during physical and chemical changes to matter. Key concepts covered are the different forms of matter, chemical and physical properties, and how energy transfers occur between chemical, thermal, electromagnetic, and electrical forms during changes to matter.