Teratology is the study of birth defects and their causes. Some key points:
- Around 5% of newborns have a detectable birth defect, though the cause is unknown for 70% of cases. Less than 1% are due to medications.
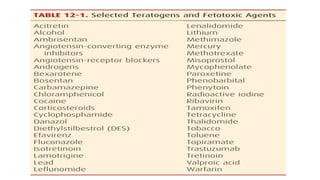
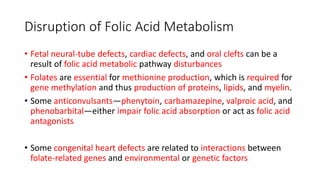
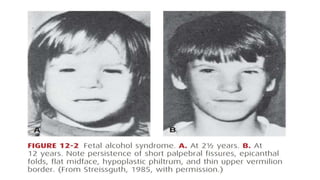
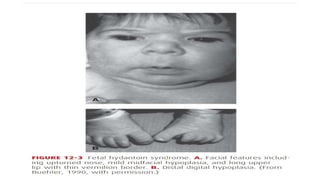
- Teratogens are agents that cause permanent changes to embryonic or fetal development, and can cause malformations (teratogen), altered growth (trophogen), or interference with organ maturation (hadegen).
- Studying teratogenicity in humans is difficult due to ethical concerns, so animal studies are also used but not definitive. Counseling women exposed to potential teratogens is important to avoid anxiety.