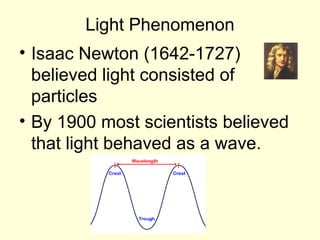
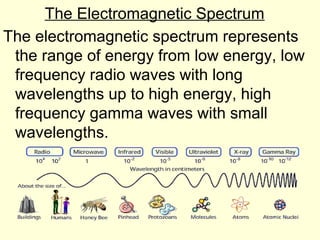
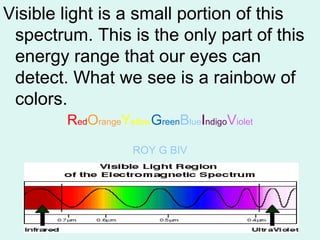
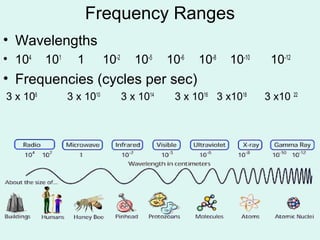
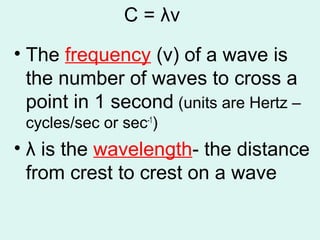
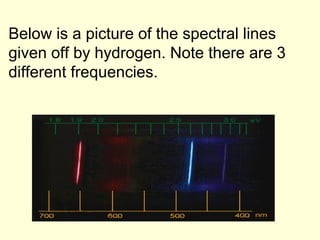
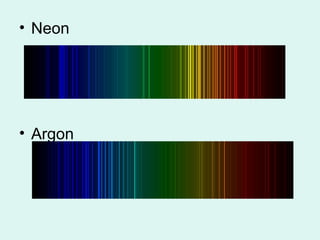
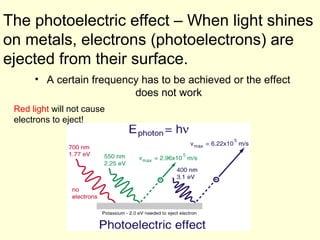
Light can be described as both a particle and a wave. The electromagnetic spectrum encompasses all types of electromagnetic radiation, including visible light, which is a small portion of the spectrum that the human eye can detect. The frequency and wavelength of electromagnetic radiation are related by the formula wavelength x frequency equals the speed of light. Analysis of the spectral lines emitted or absorbed by elements can be used to identify elements and determine properties of stars and other celestial objects.