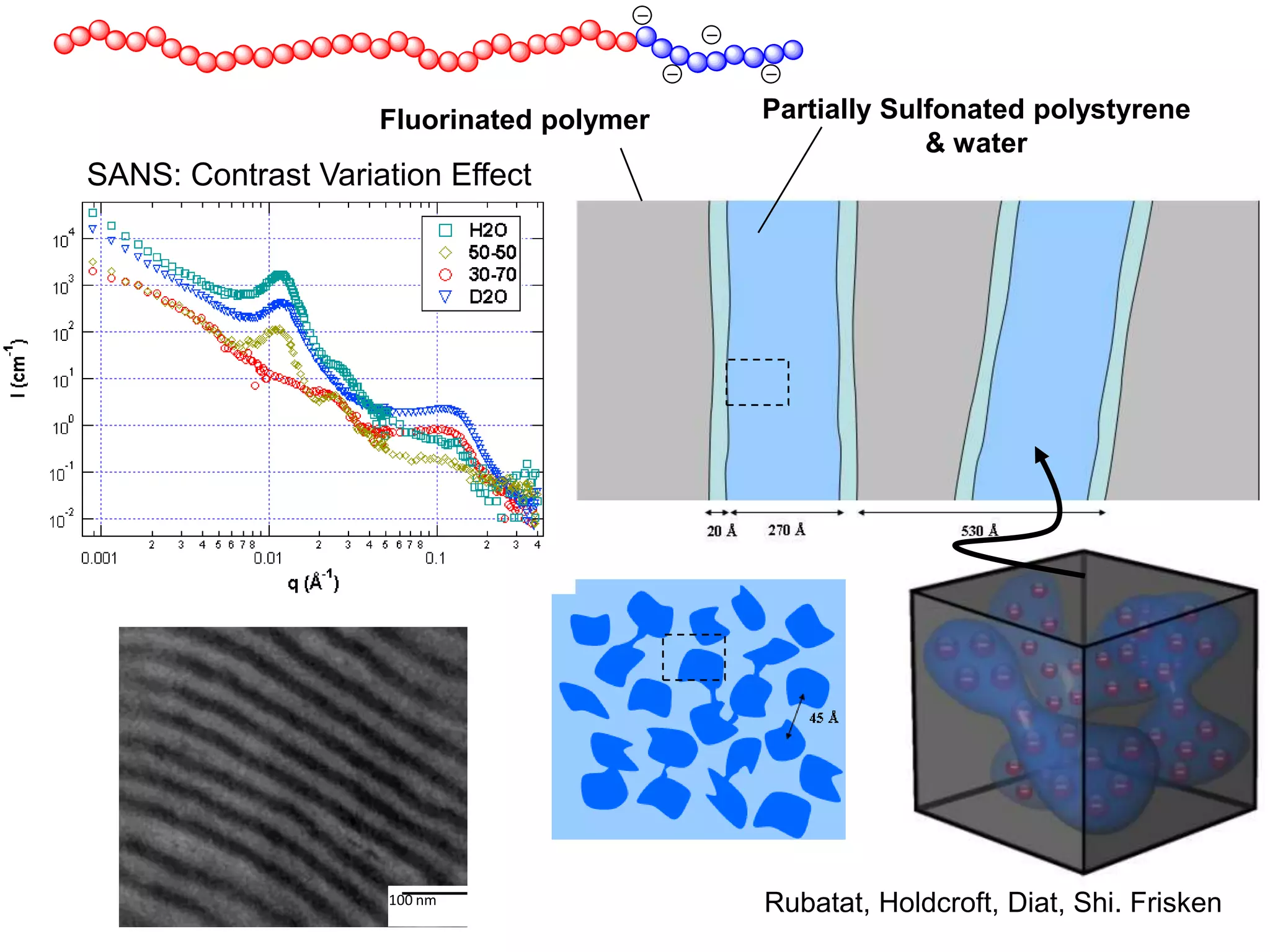
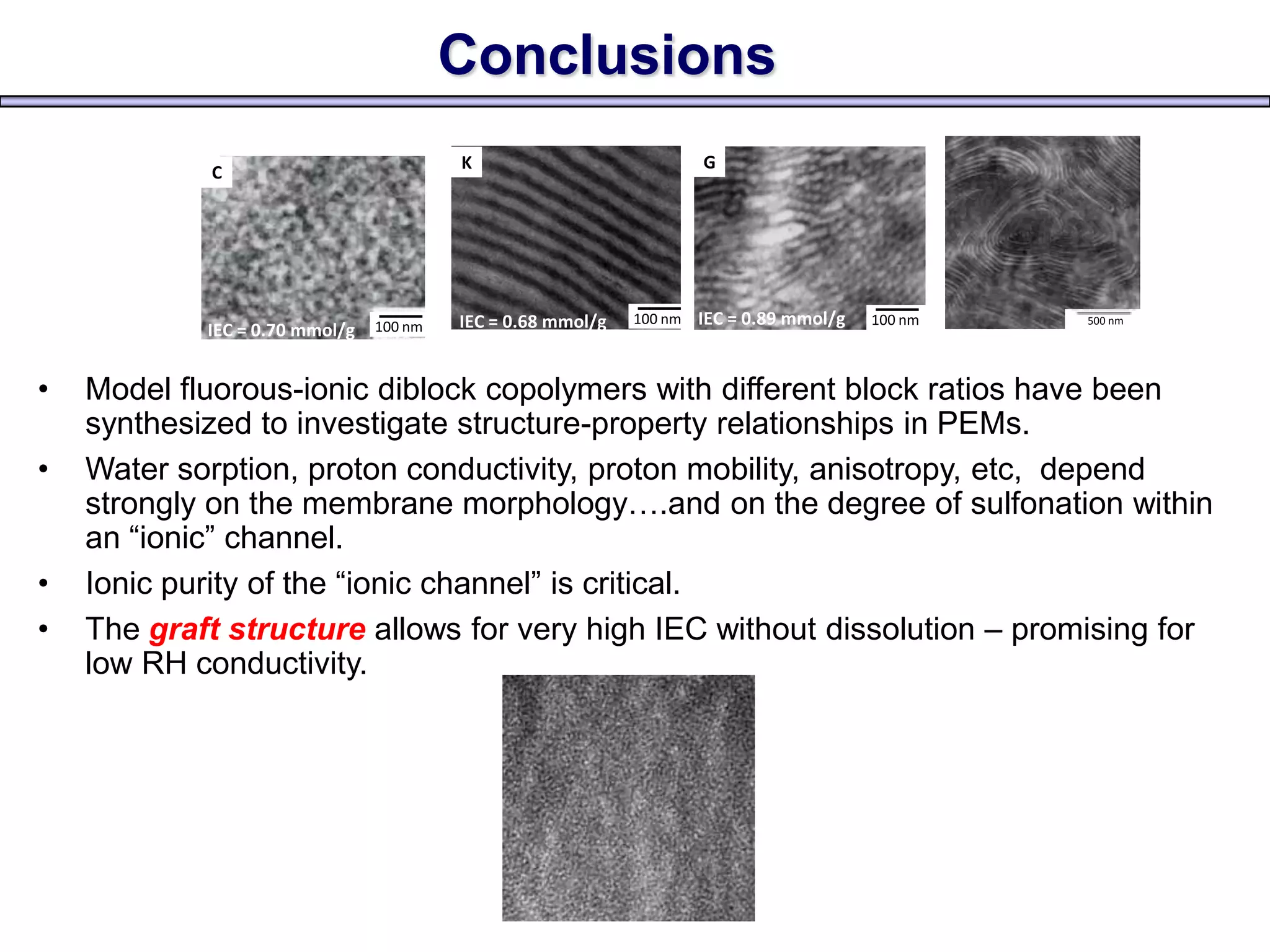
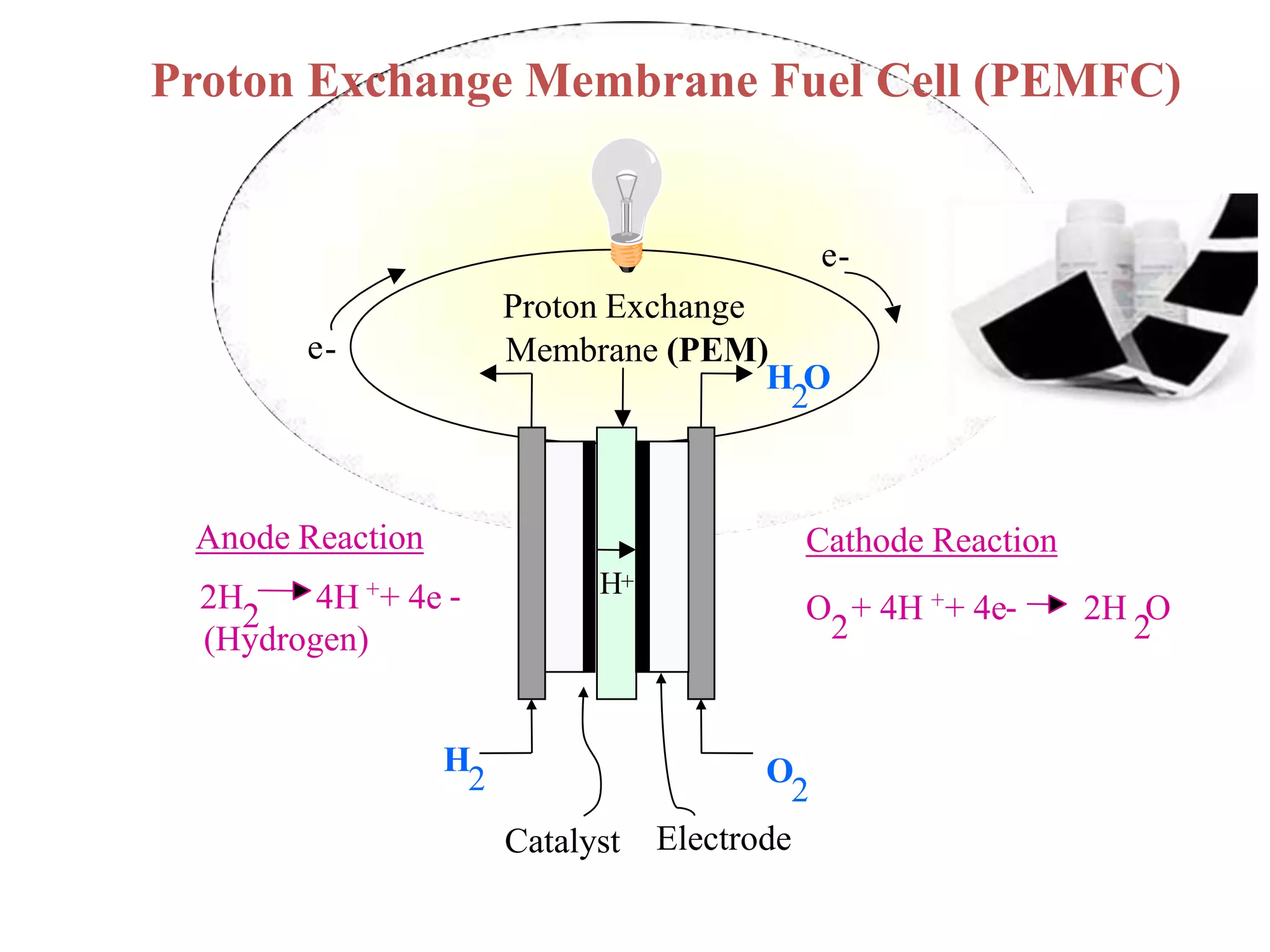
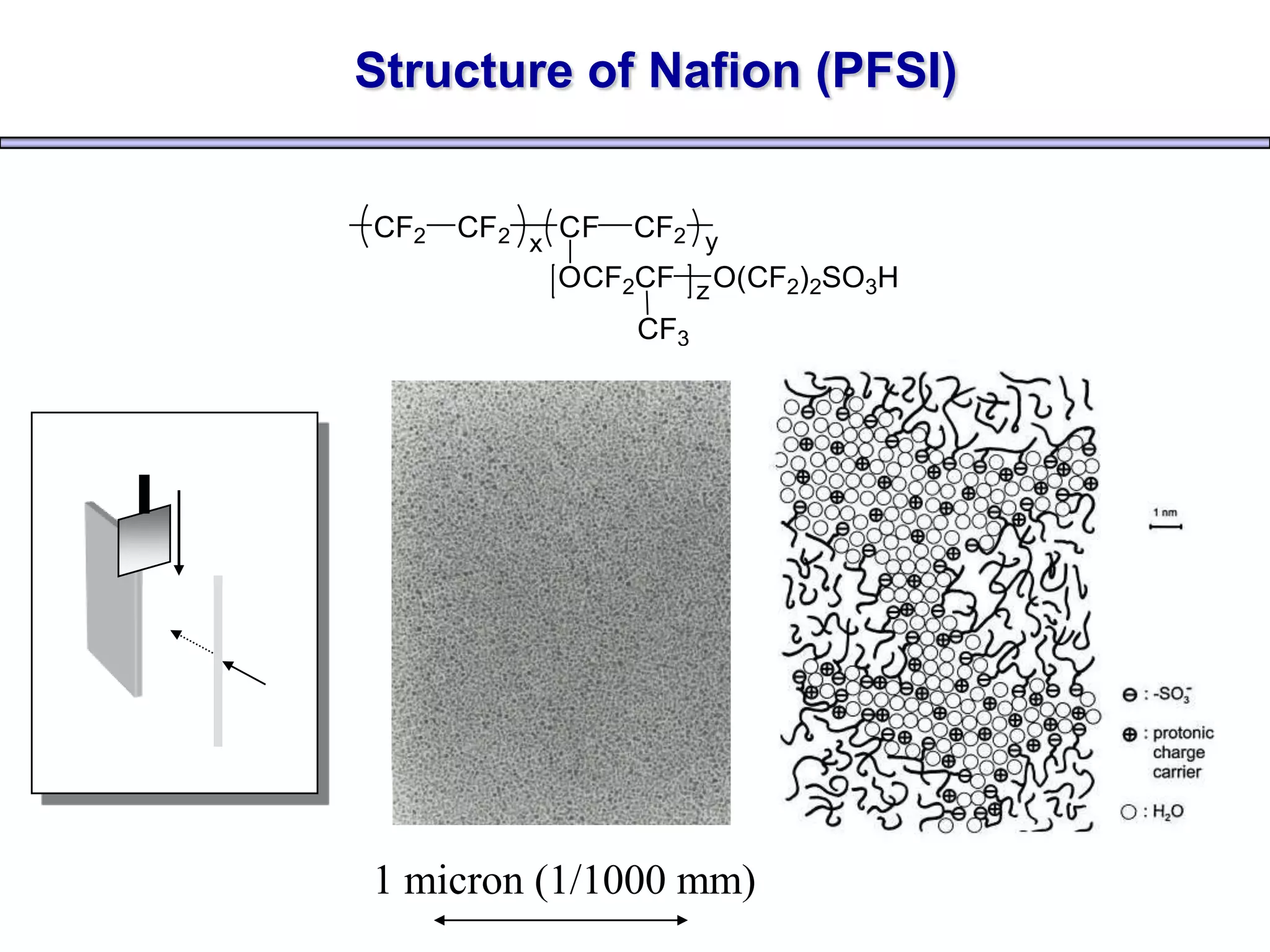
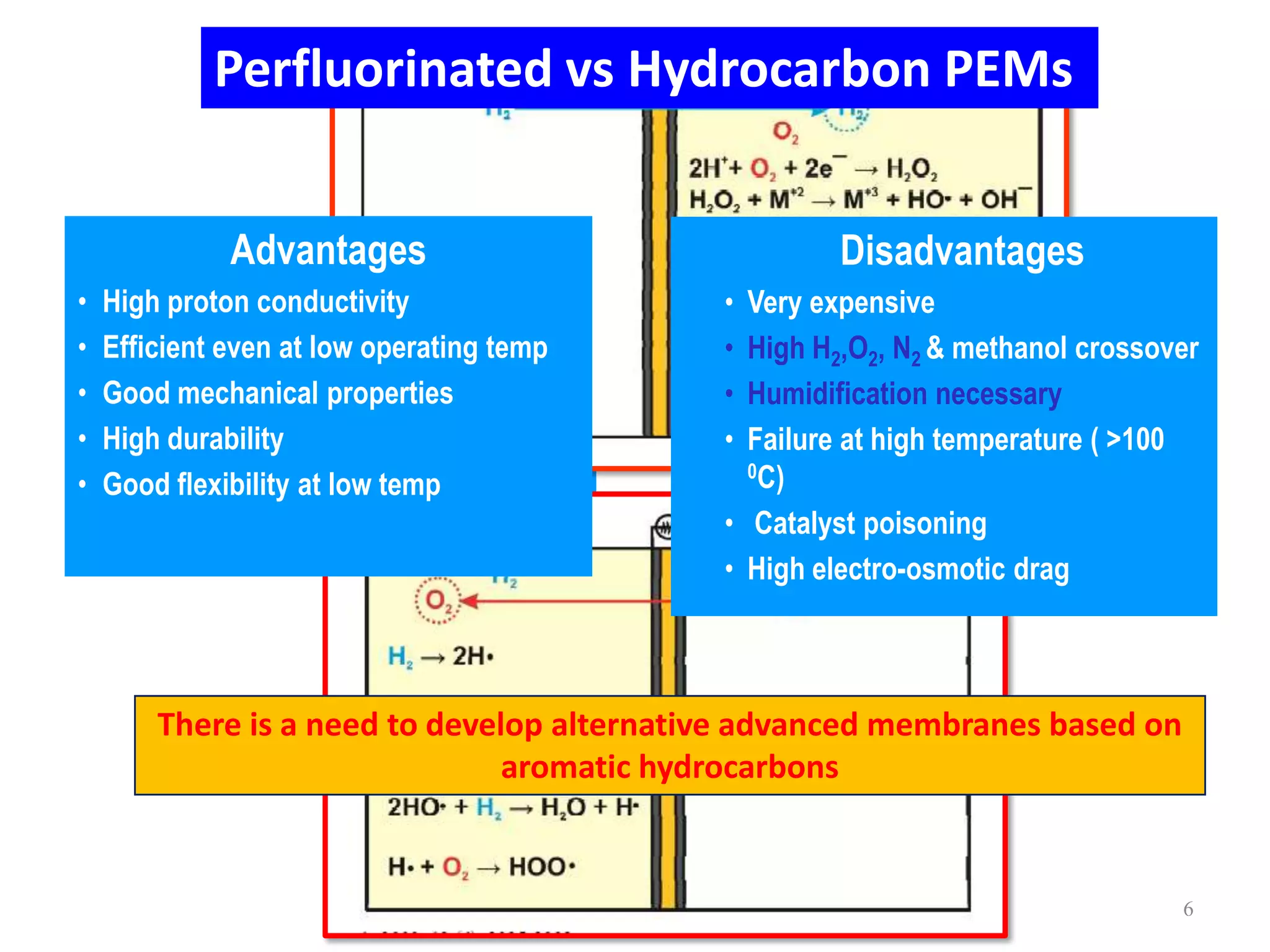
Simon Fraser University researchers developed novel polymer membranes for fuel cells. They synthesized fluoropolymer-block-ionic and fluorous-ionic graft copolymers with different architectures. The membrane morphology, including ionic channel size and continuity, depended on polymer structure and affected properties like water uptake and proton conductivity. Fully sulfonated membranes showed continuous increases in proton conductivity with ion exchange capacity, while partially sulfonated membranes peaked at moderate capacities due to conductivity drops at high capacities. The graft structure allowed high capacities without dissolution, promising for low-humidity proton conductivity needed in fuel cells.







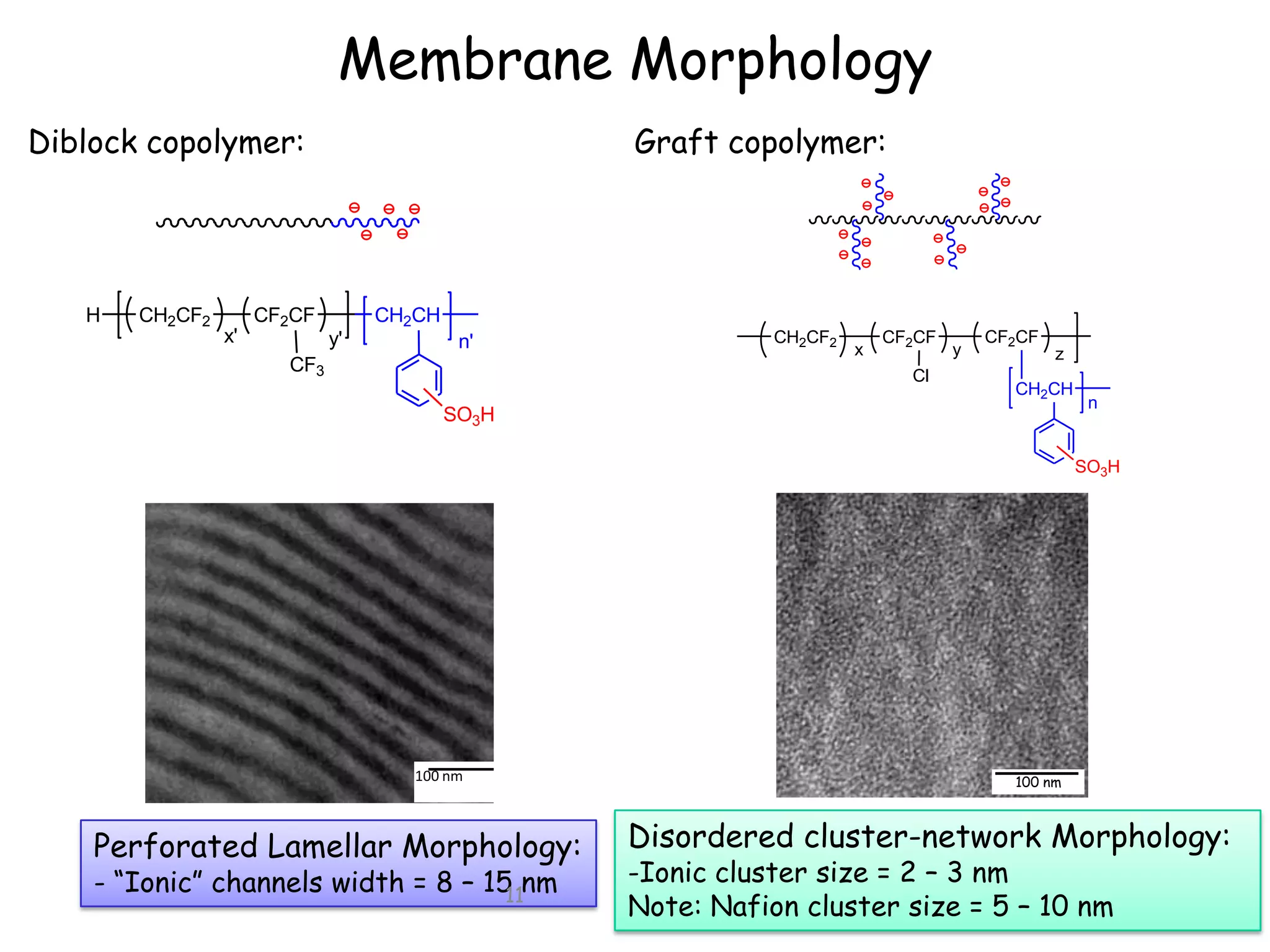
![Diblock vs Graft Membrane
Diblock copolymer:
Graft copolymer:
100 nm
100 nm
-
120
90
60
30
0
0.08
0.06
0.04
0.02
0.00
0.0
0.5
1.0
1.5
2.0
2.5
IEC (mmol/g)
Grafts (small ionic clusters):
0.0
0.5
1.0
1.5
2.0
IEC (mmol/g)
- Less water swelling Lower proton mobility
- Maintain good mechanical property and high
proton concentrations
2.5
1.2
2.5
1.0
2.0
0.8
1.5
0.6
1.0
0.4
0.5
0.2
0.0
x 103 (cm2 s-1 V-1)
dissolves
150
3.0
eff
Proton Conductivity (S/cm)
0.10
0.0
0.0
0.5
1.0
1.5
2.0
2.5
IEC (mmol/g)
Diblocks (long-range channels):
- Greater water uptake Higher proton
conductivity and mobility
- Excessive water swelling mechanical
instability and limited attainable IEC.
[H+] (M)
H2O]/[SO3 ])
180](https://image.slidesharecdn.com/325steevn-131211235220-phpapp02/75/325-steevn-12-2048.jpg)