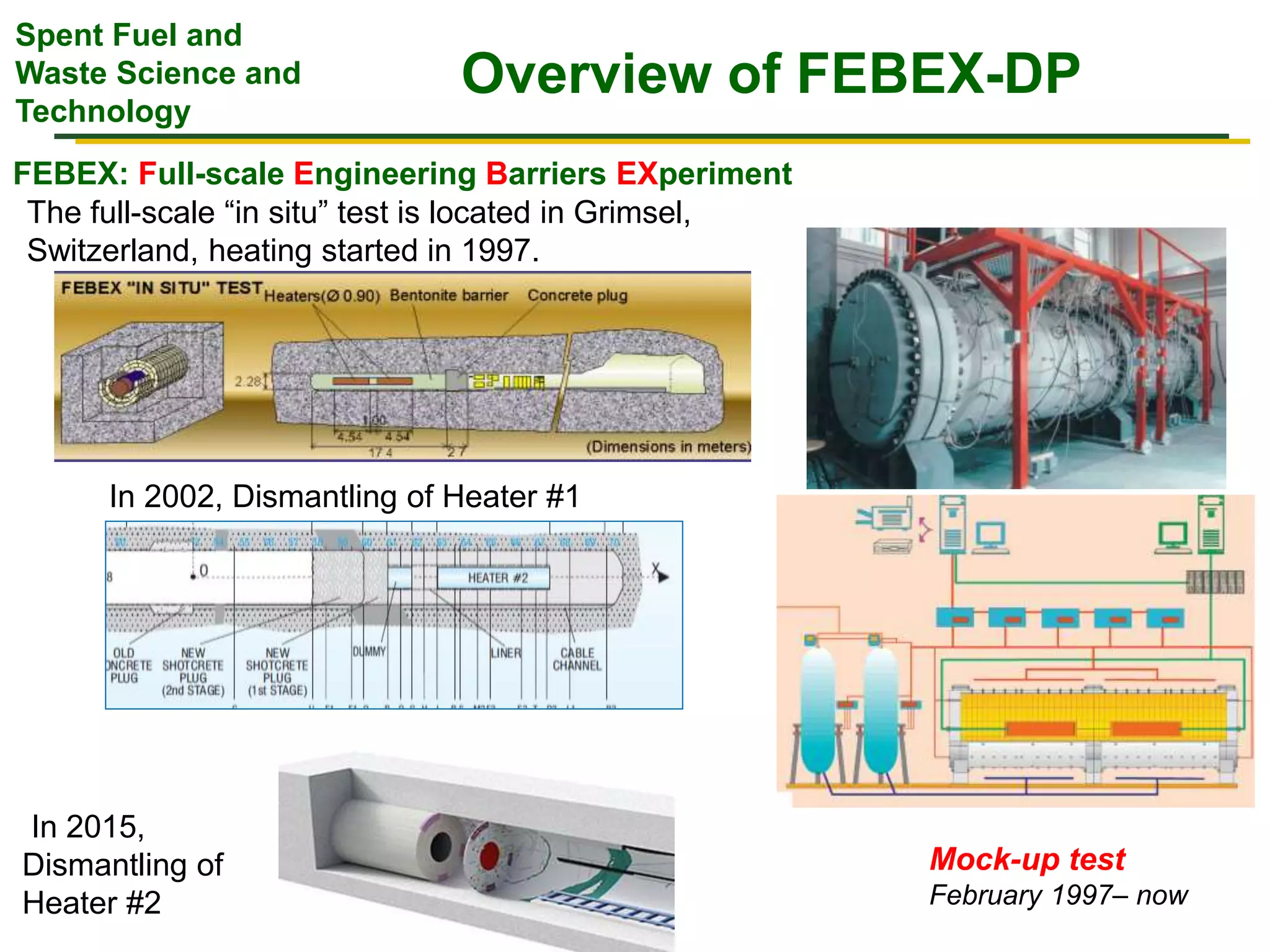
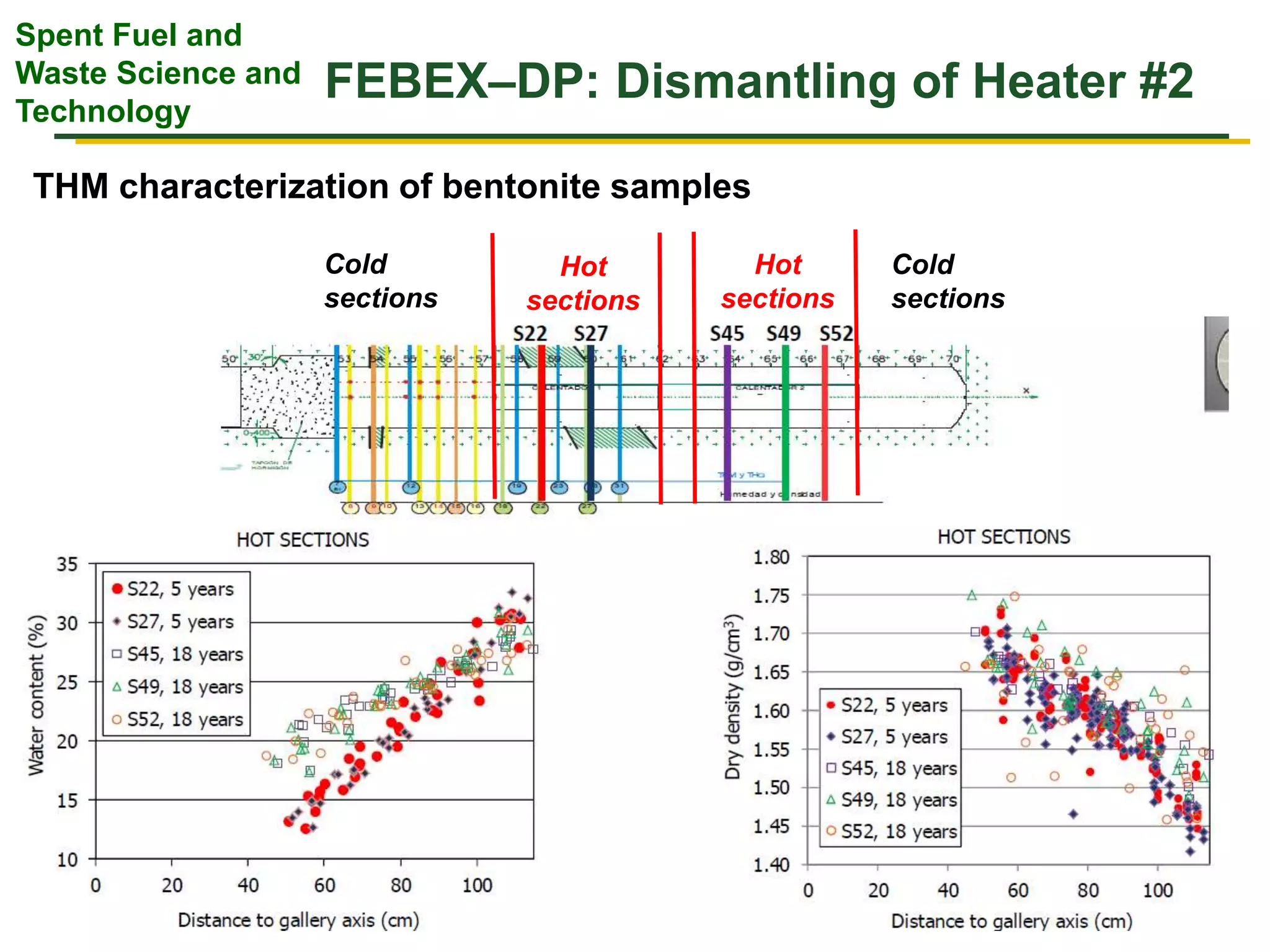
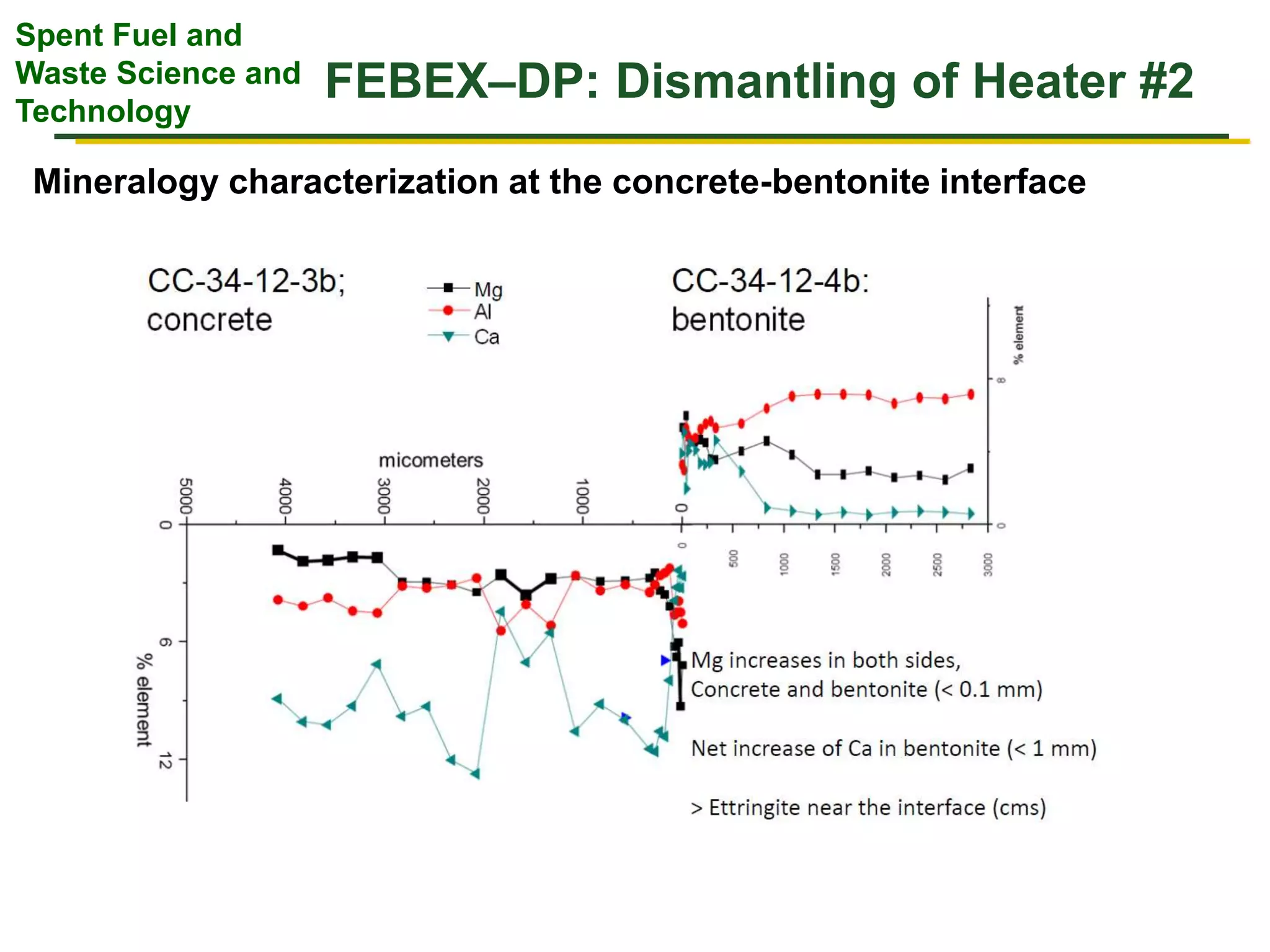
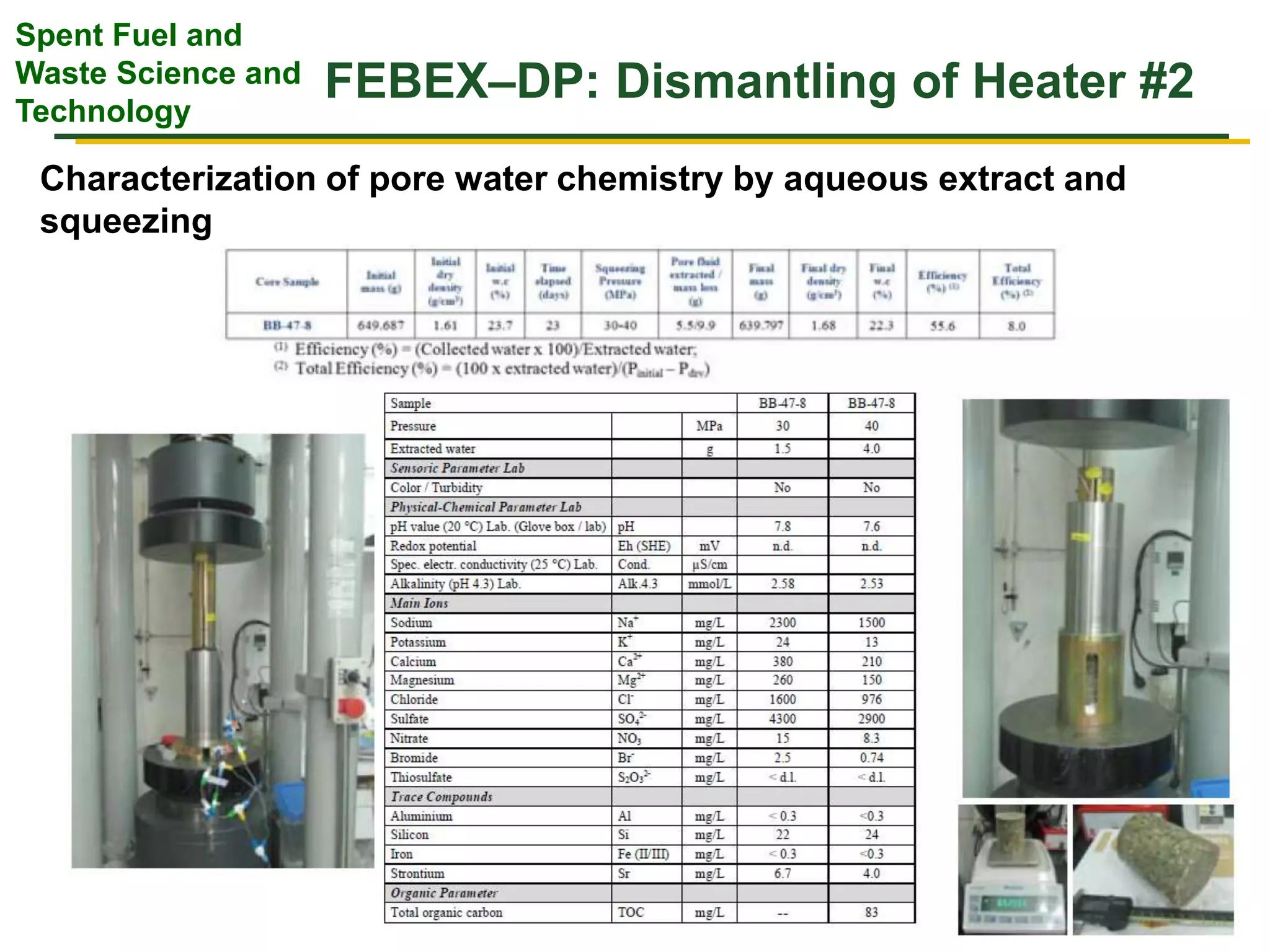
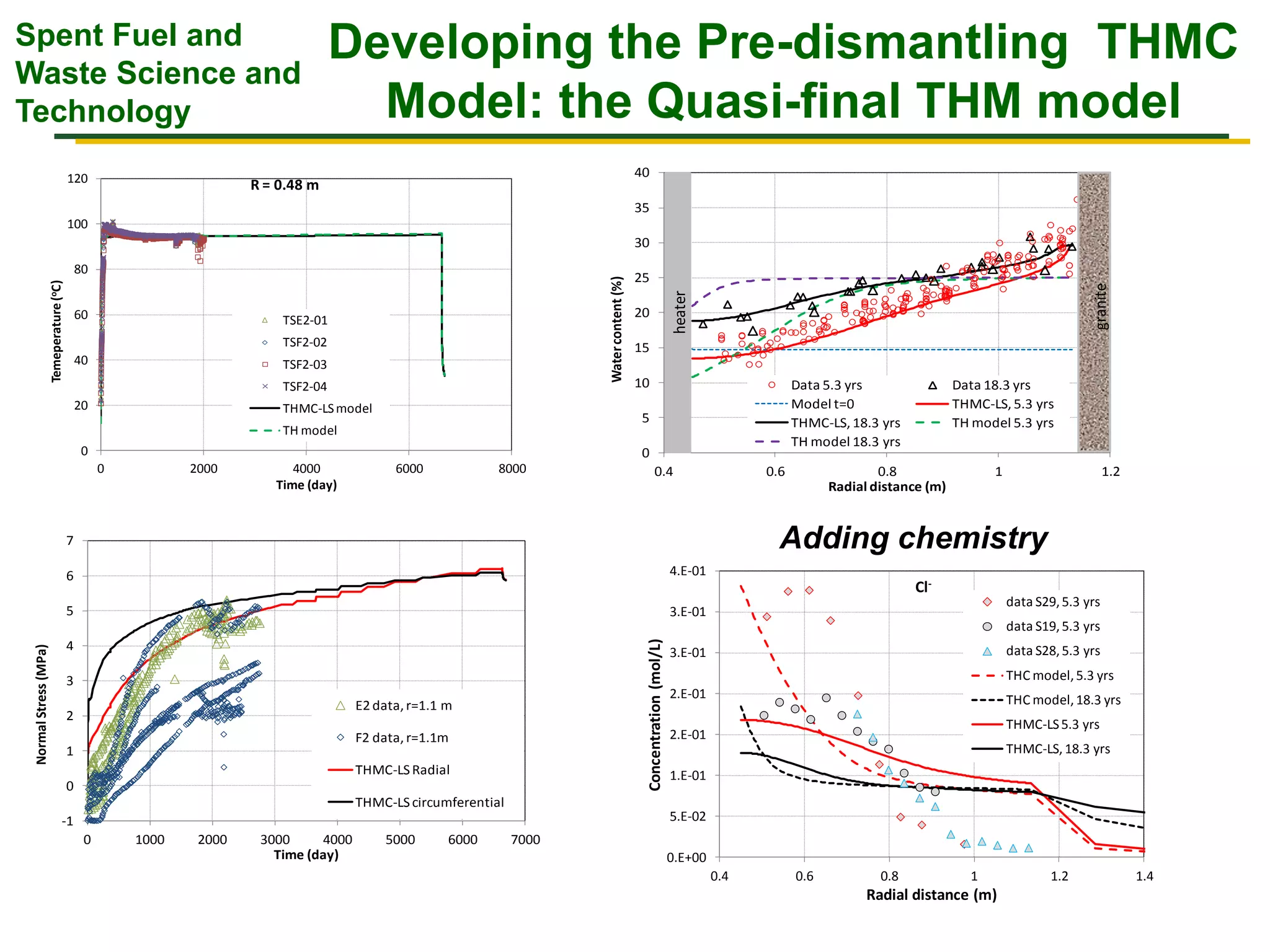
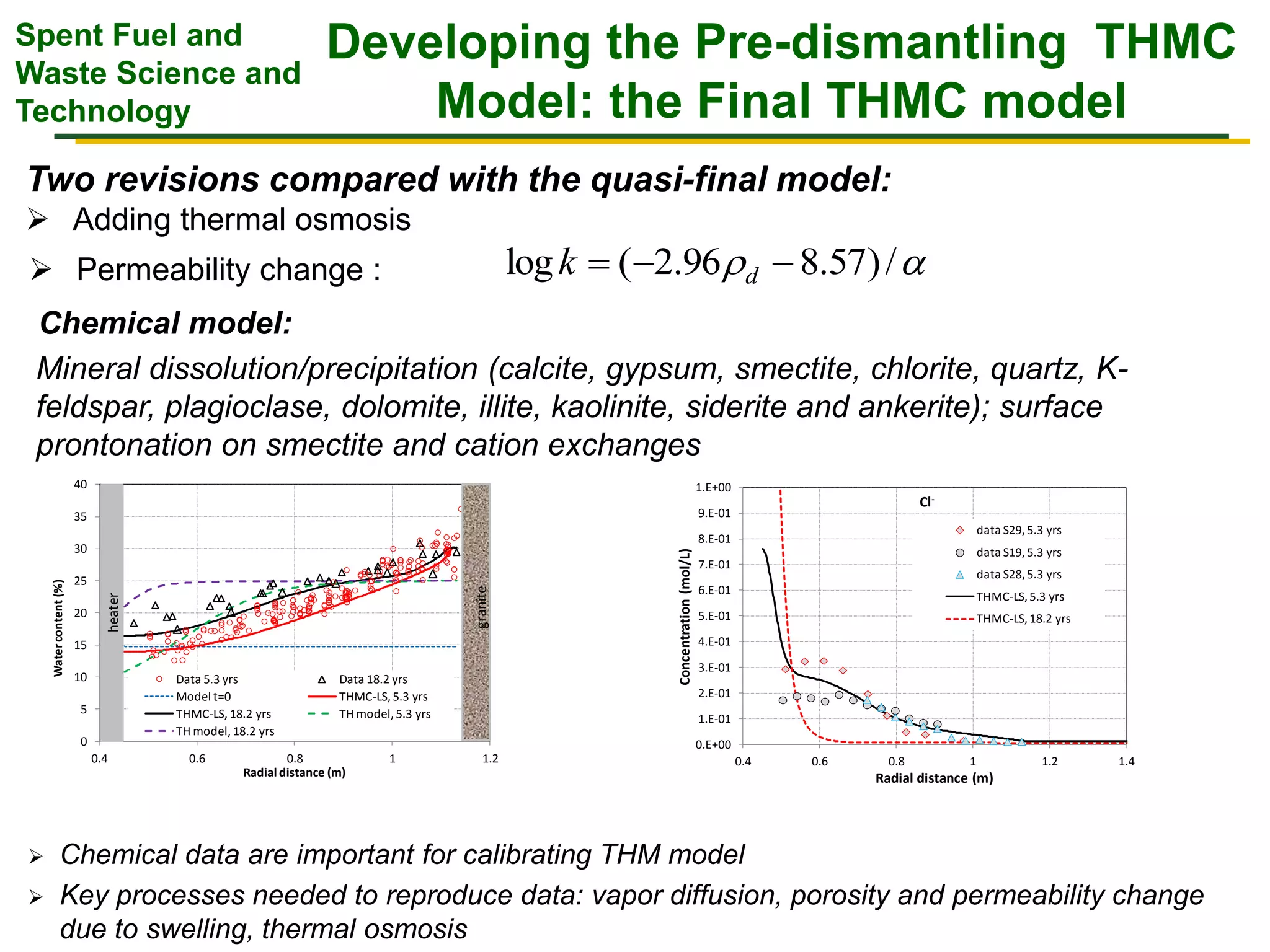
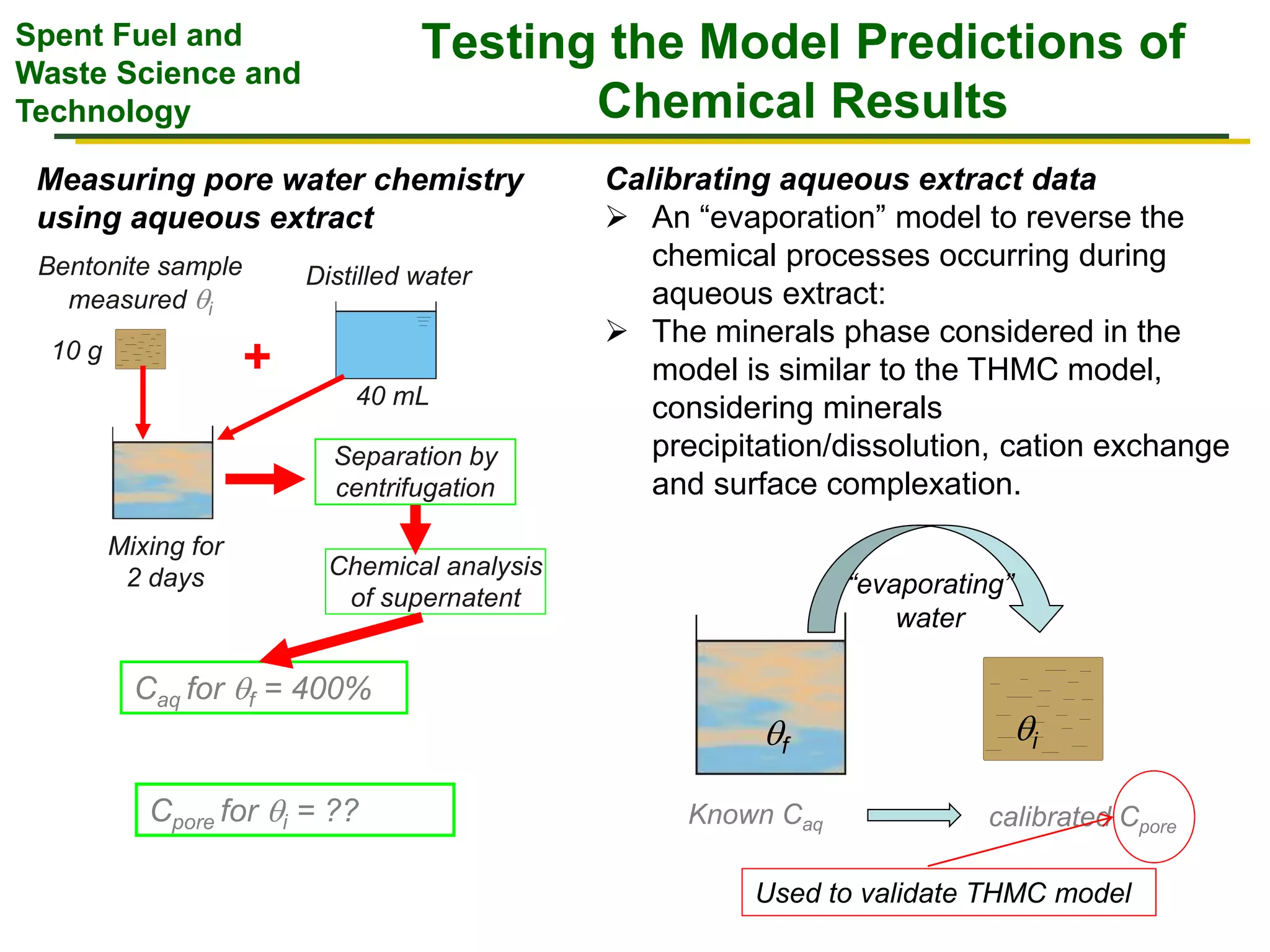
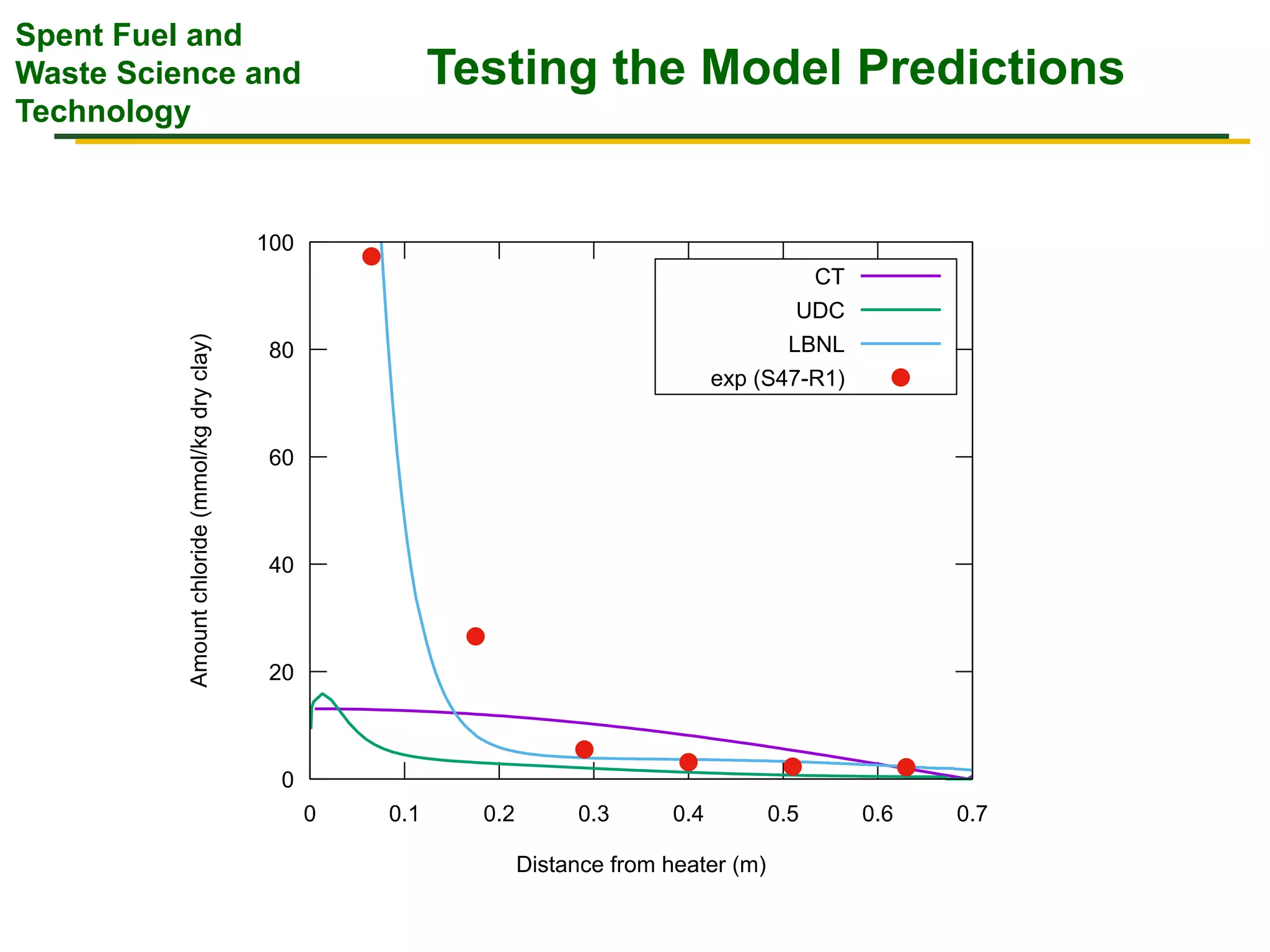
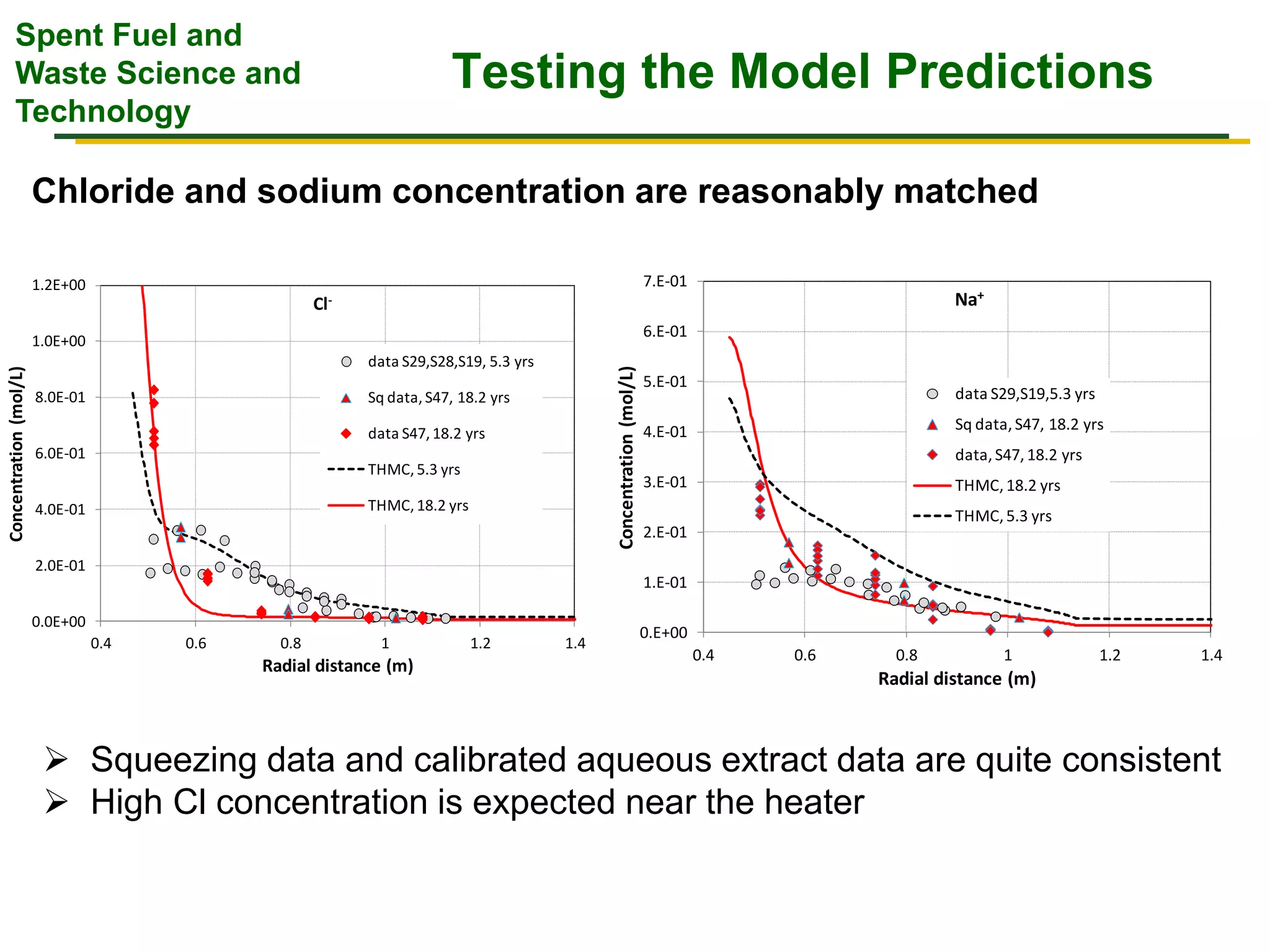
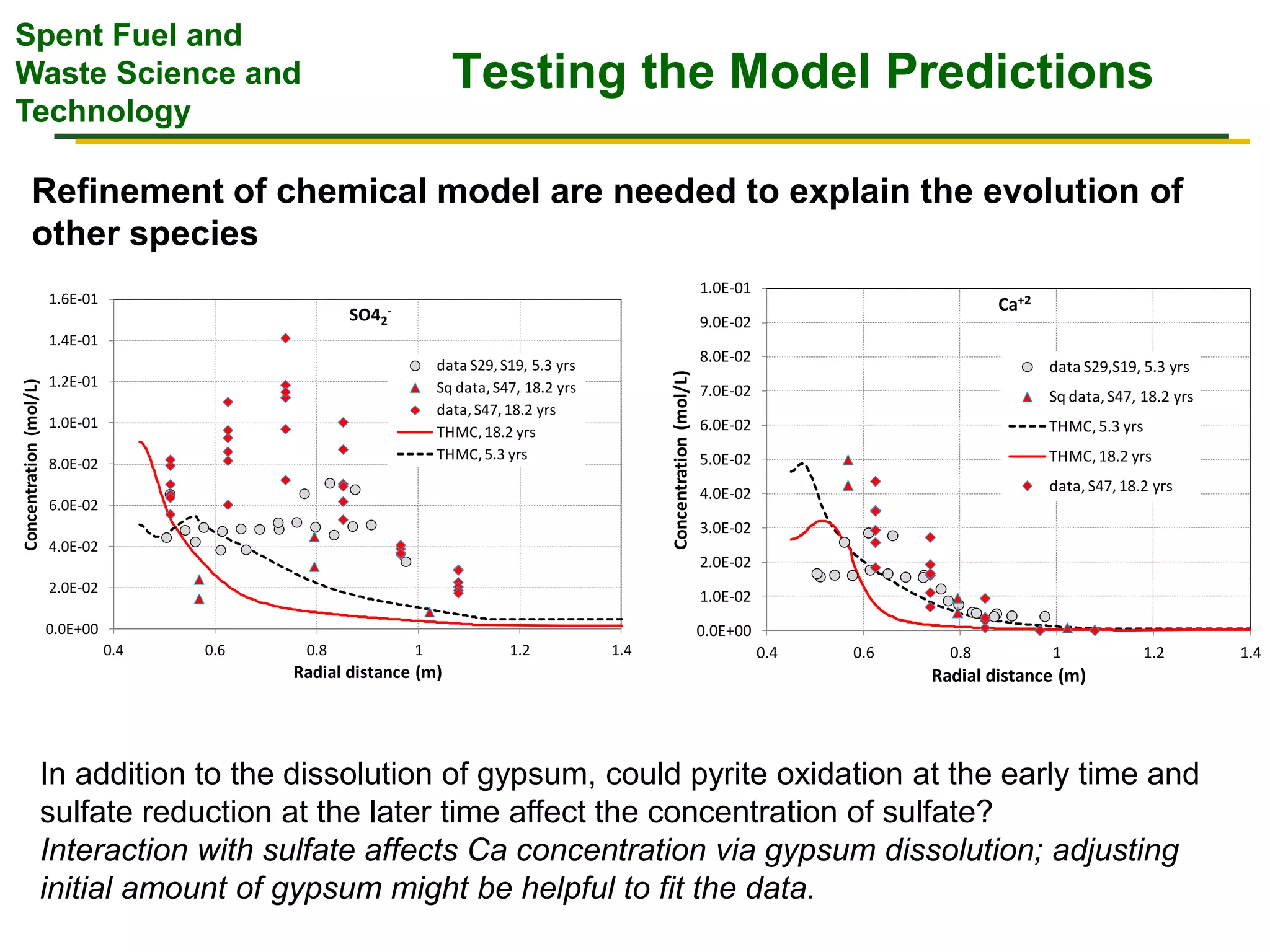
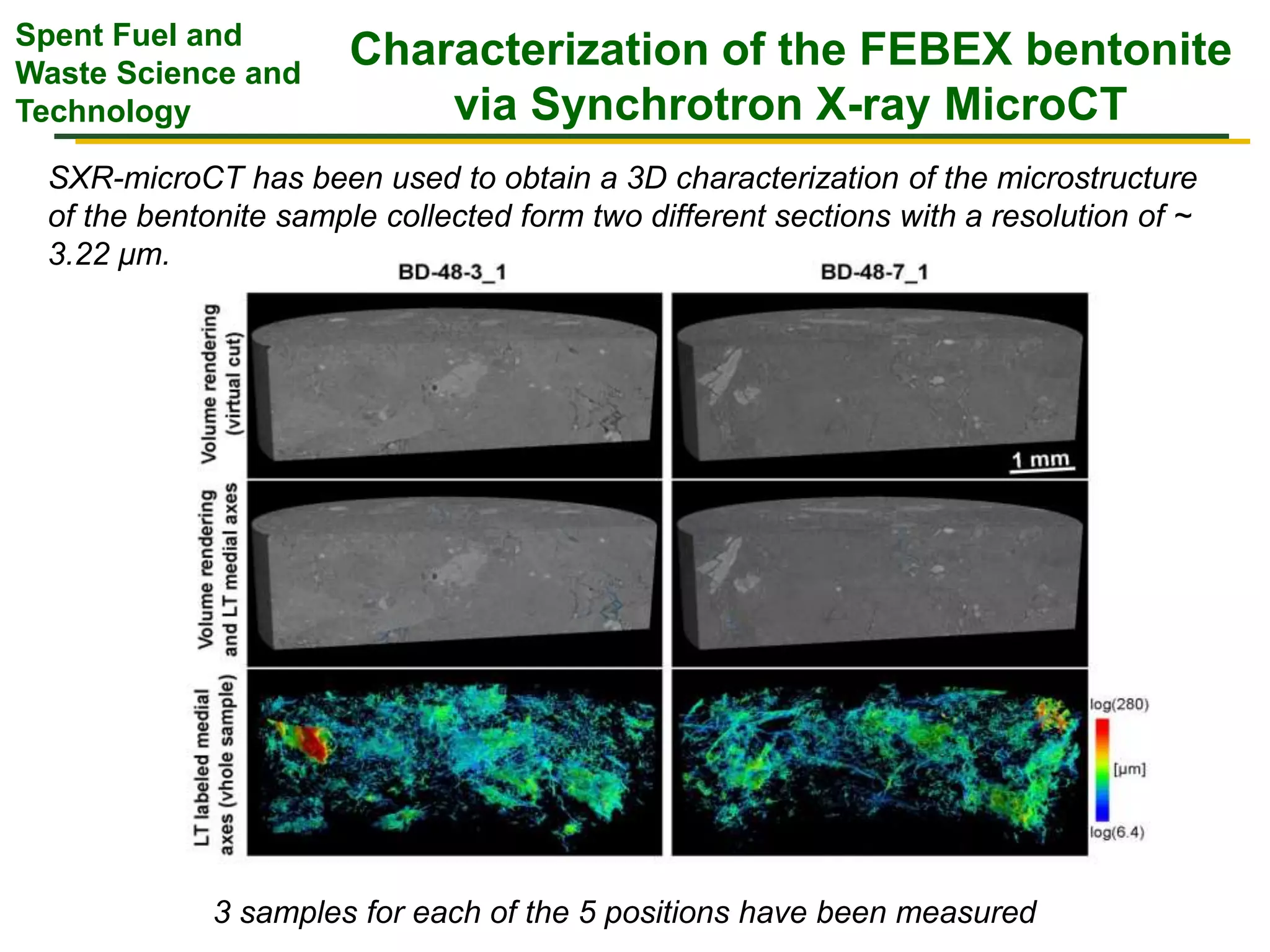
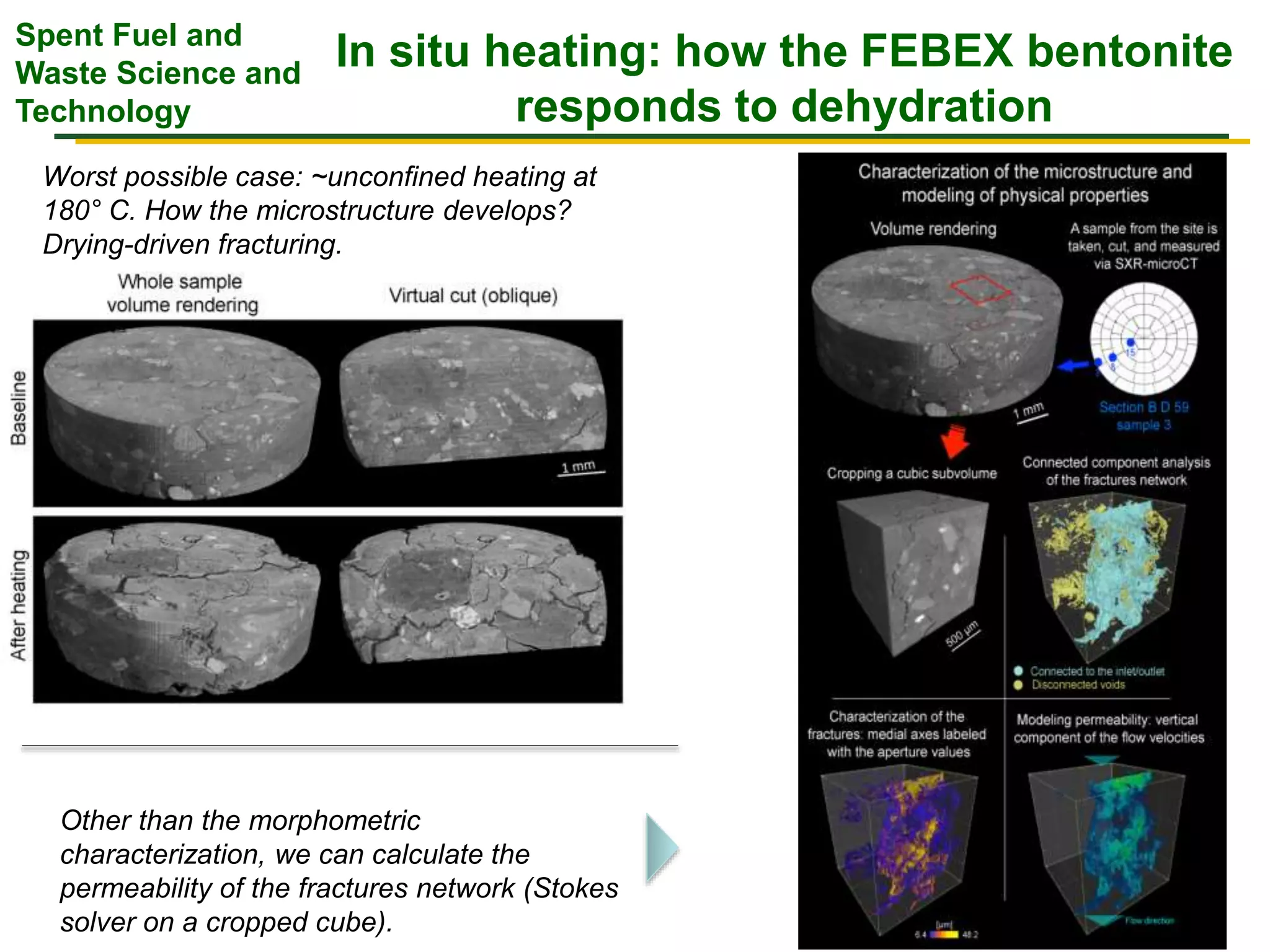
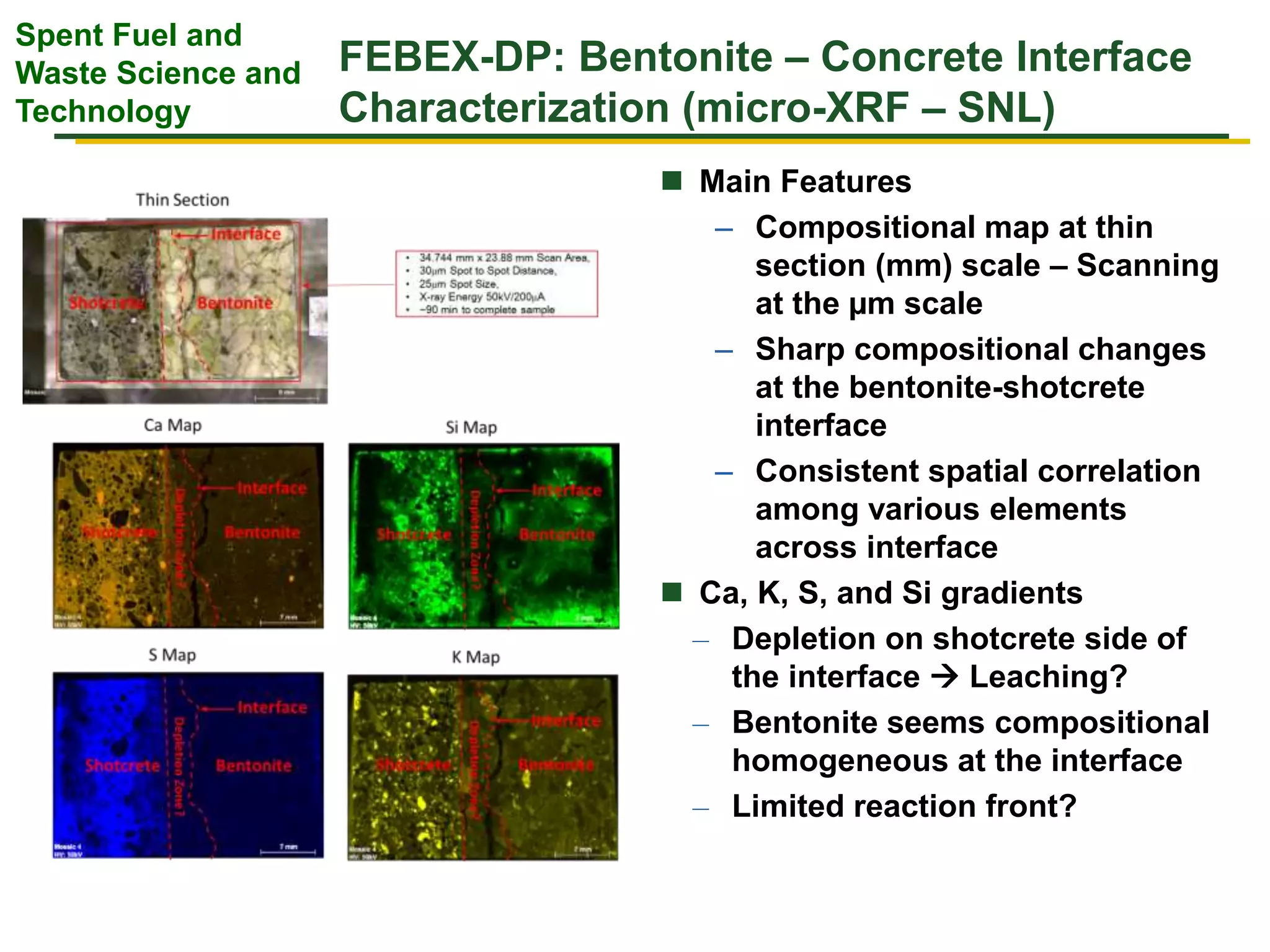
The document summarizes research from the FEBEX-DP collaboration studying the full-scale FEBEX bentonite experiment located in Grimsel, Switzerland. It discusses characterization of the bentonite following dismantling of heater #2, including changes to density, water content, mineralogy, and pore water chemistry. THM and THMC models were developed and validated against experimental data to analyze thermal-hydrological-mechanical and chemical processes. Microstructural analysis using synchrotron X-ray microCT provided 3D characterization of the bentonite microstructure. Further work is focused on refining chemical models, analyzing gas evolution and microbial activity, and characterizing the bentonite-concrete interface.