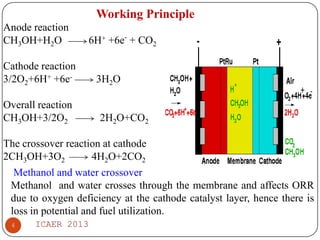
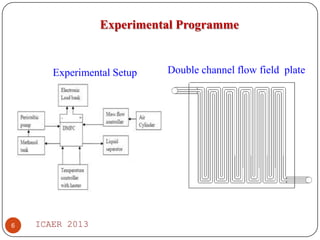
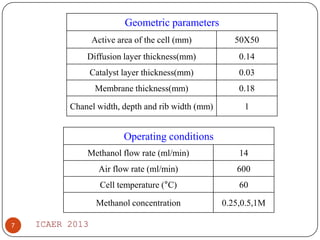
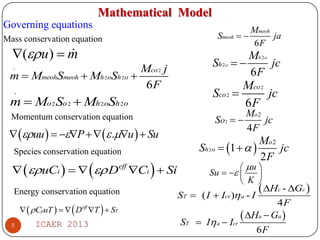
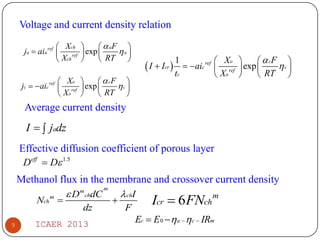
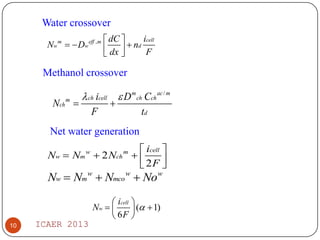
The document summarizes heat and mass transfer characteristics of direct methanol fuel cells (DMFCs) based on experiments and modeling. Key points:
- A 3D non-isothermal model is developed to predict methanol and temperature distributions in the anode. Experimental results validate the model.
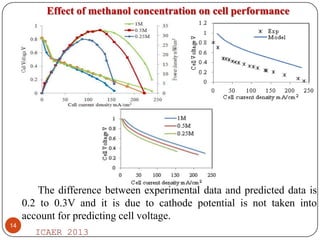
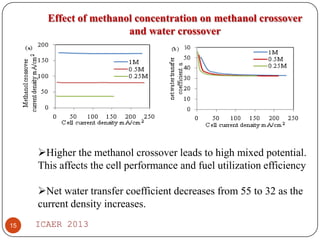
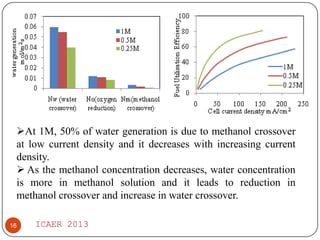
- Increasing methanol concentration does not significantly impact net water generation but does increase methanol crossover, affecting cell performance.
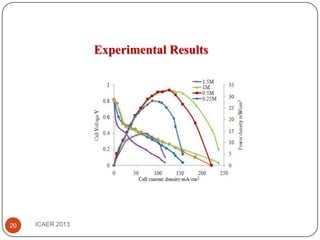
- At 1M methanol concentration and 230mA/cm2 current density, the fuel utilization efficiency is 57% despite high methanol crossover.
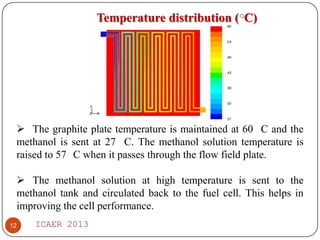
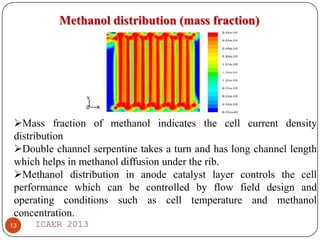
- Temperature distribution shows methanol solution heated to 57°C from 27°C, improving cell performance. Double channel serpentine flow field aids methanol diffusion.