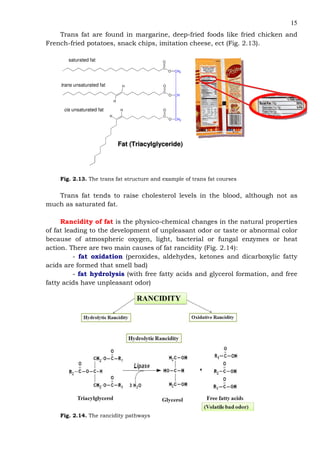
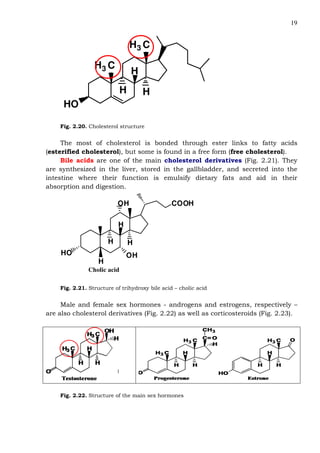
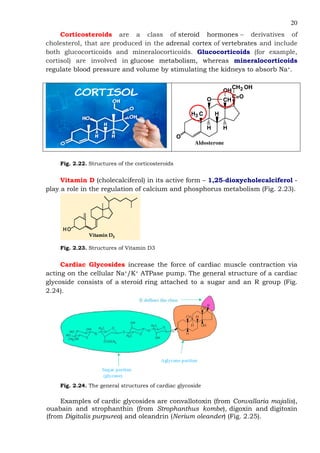
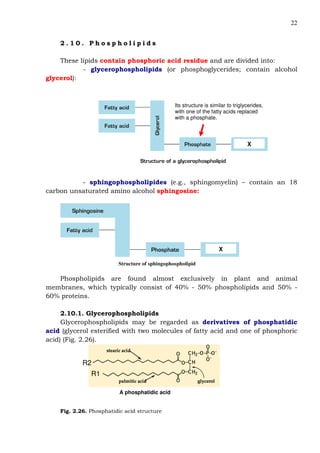
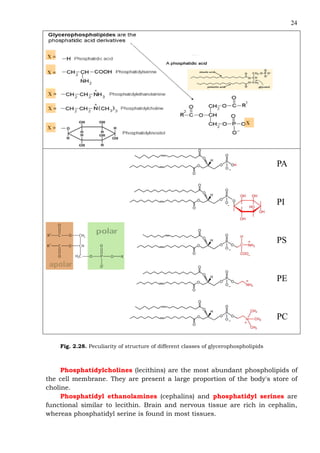
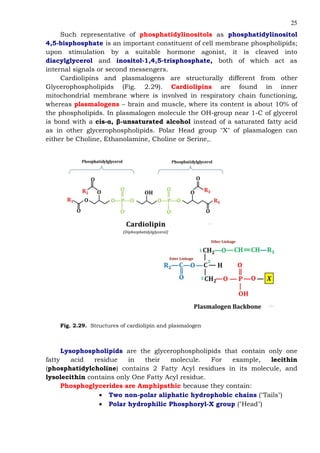
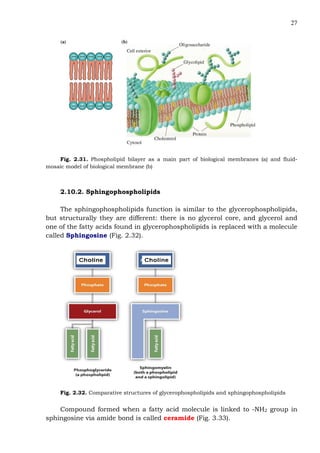
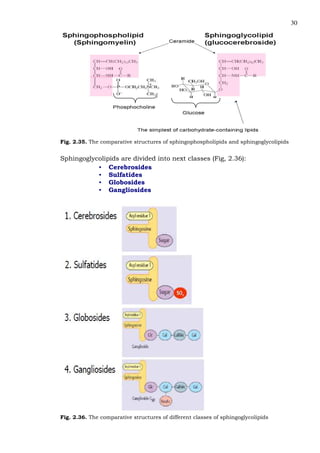
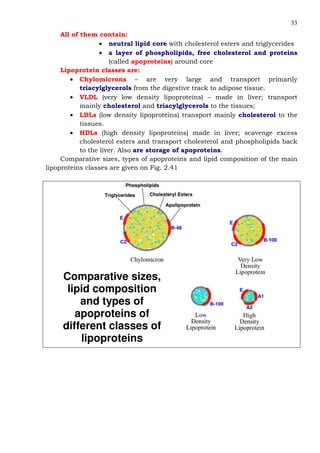
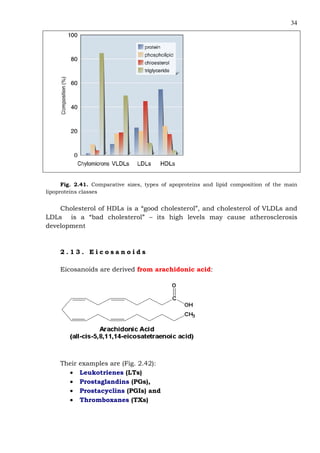
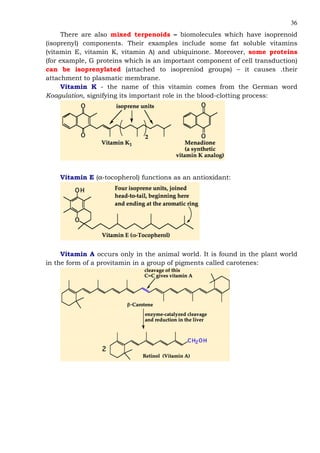
This document provides an overview of lipids and fatty acids. It begins with general information about lipids, including their classification as simple or complex lipids. Fatty acids are then discussed in more detail, including their structure, classification as saturated, monounsaturated, or polyunsaturated, and various systems for naming fatty acids from systematic to shorthand nomenclature. The document also covers lipid derivatives and uses of lipids in the body before concluding with test questions.