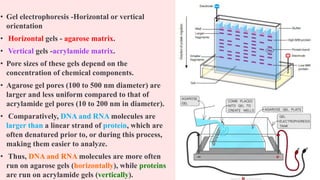
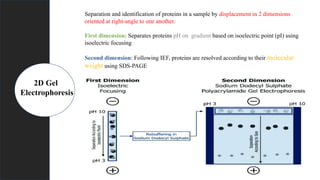
The document provides an overview of 2D gel electrophoresis, explaining its principles, types, workflow, and applications in protein separation based on isoelectric point and molecular weight. It details the process including isoelectric focusing, SDS-PAGE, and subsequent analysis techniques like mass spectrometry. Additionally, it discusses the limitations of traditional 1D electrophoresis and how 2D techniques improve resolution in proteomic studies.