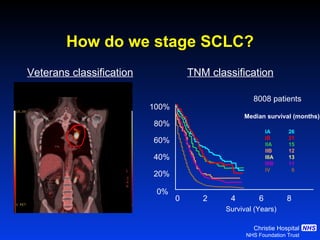
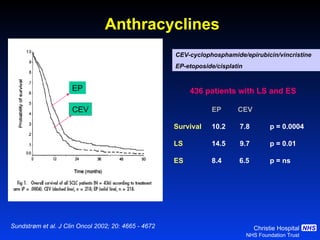
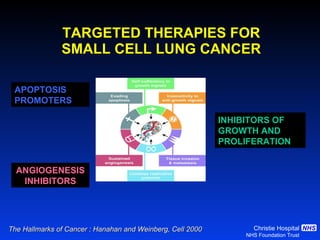
- Small cell lung cancer (SCLC) has seen little therapeutic advancement in over 20 years. Platinum-etoposide remains the standard chemotherapy regimen.
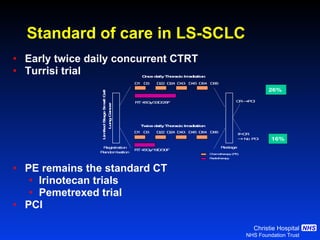
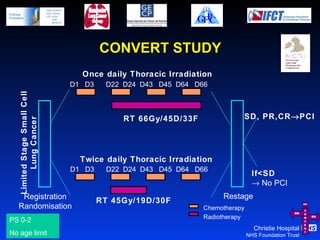
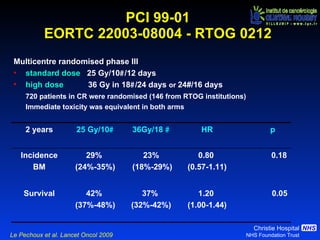
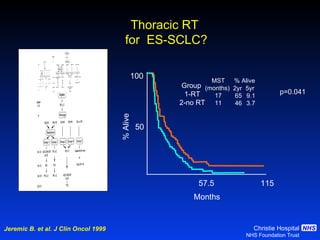
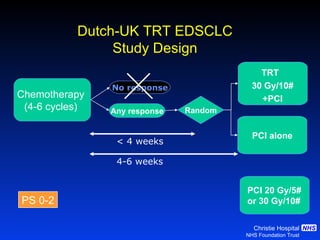
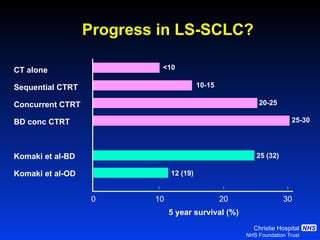
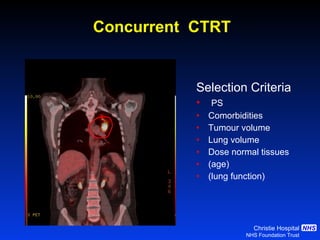
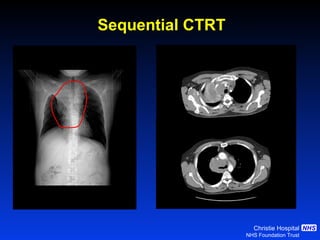
- For limited-stage SCLC, concurrent chemoradiation is the standard of care and provides better outcomes than sequential treatment. Early twice-daily radiotherapy with prophylactic cranial irradiation improves survival.
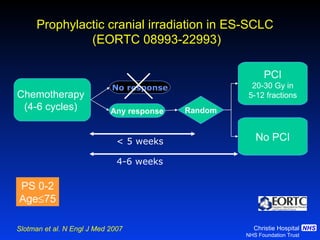
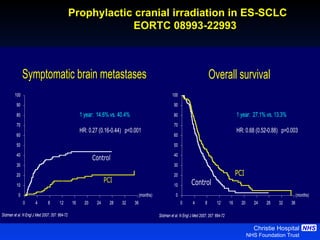
- For extensive-stage SCLC, prophylactic cranial irradiation after chemotherapy reduces the risk of brain metastases and improves survival compared to no cranial irradiation.







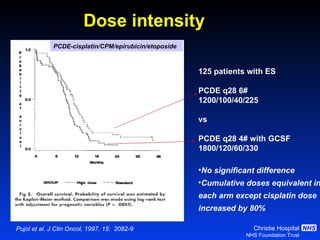
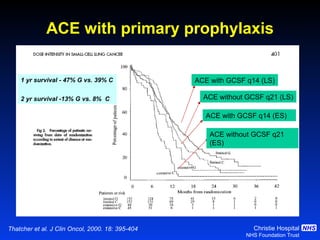

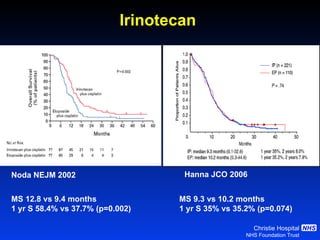
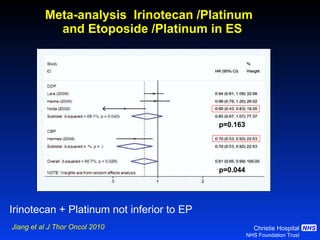
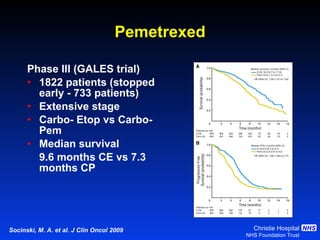
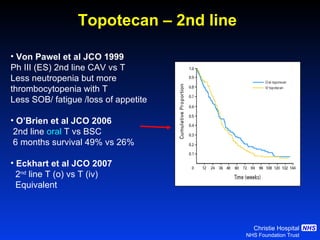

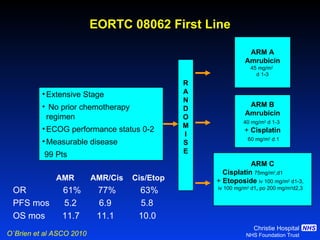
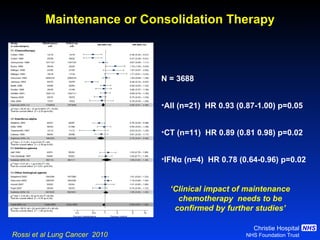

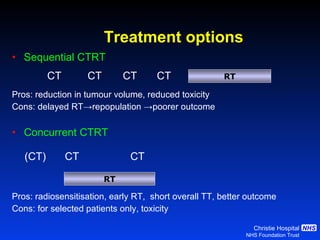
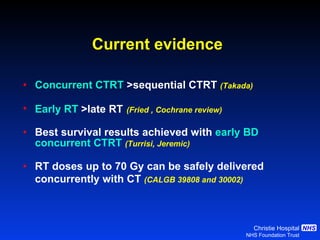
![Timing of thoracic RT with chemotherapy J Clin Oncol. 2004;22:4837-45 7 RCTs Advantage of early (<9 weeks) radiotherapy 2 yr % NNT for benefit P All (1524) Platinum Platinum+ HART +5.2 [0.6-9.7] 20 0.03 +9.8 [3.8-15.9] 10 0.001 +16.7 [9.4-26] 6 0.001](https://image.slidesharecdn.com/28faivre-finnsclc-110414025424-phpapp02/85/MCO-2011-Slide-28-C-Faivre-Finn-SCLC-29-320.jpg)