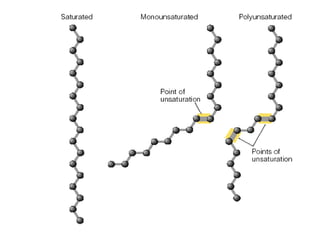
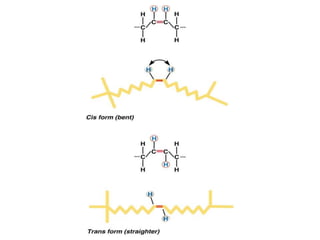
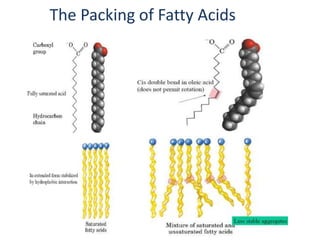
This document discusses fatty acids, which are carboxylic acids and key building blocks for lipids. Fatty acids can be saturated or unsaturated depending on whether they contain single or double carbon bonds. They are classified based on chain length and the number and position of double bonds. Unsaturated fatty acids have lower melting points than saturated fatty acids. Essential fatty acids like omega-3 and omega-6 must be obtained through diet as the human body cannot synthesize them. Fatty acids play important structural and physiological roles.