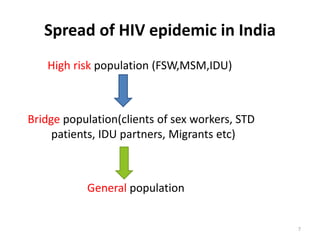
HIV/AIDS is caused by the HIV virus which weakens the immune system. It is transmitted through unprotected sex, contaminated blood, sharing needles, and from mother to child during pregnancy, delivery, or breastfeeding. As the virus progresses it can cause opportunistic infections and cancers. India has a large population affected due to transmission among high risk groups like sex workers, clients, and drug users. Prevention methods aim to reduce transmission through awareness, testing, treatment, and vaccination programs.