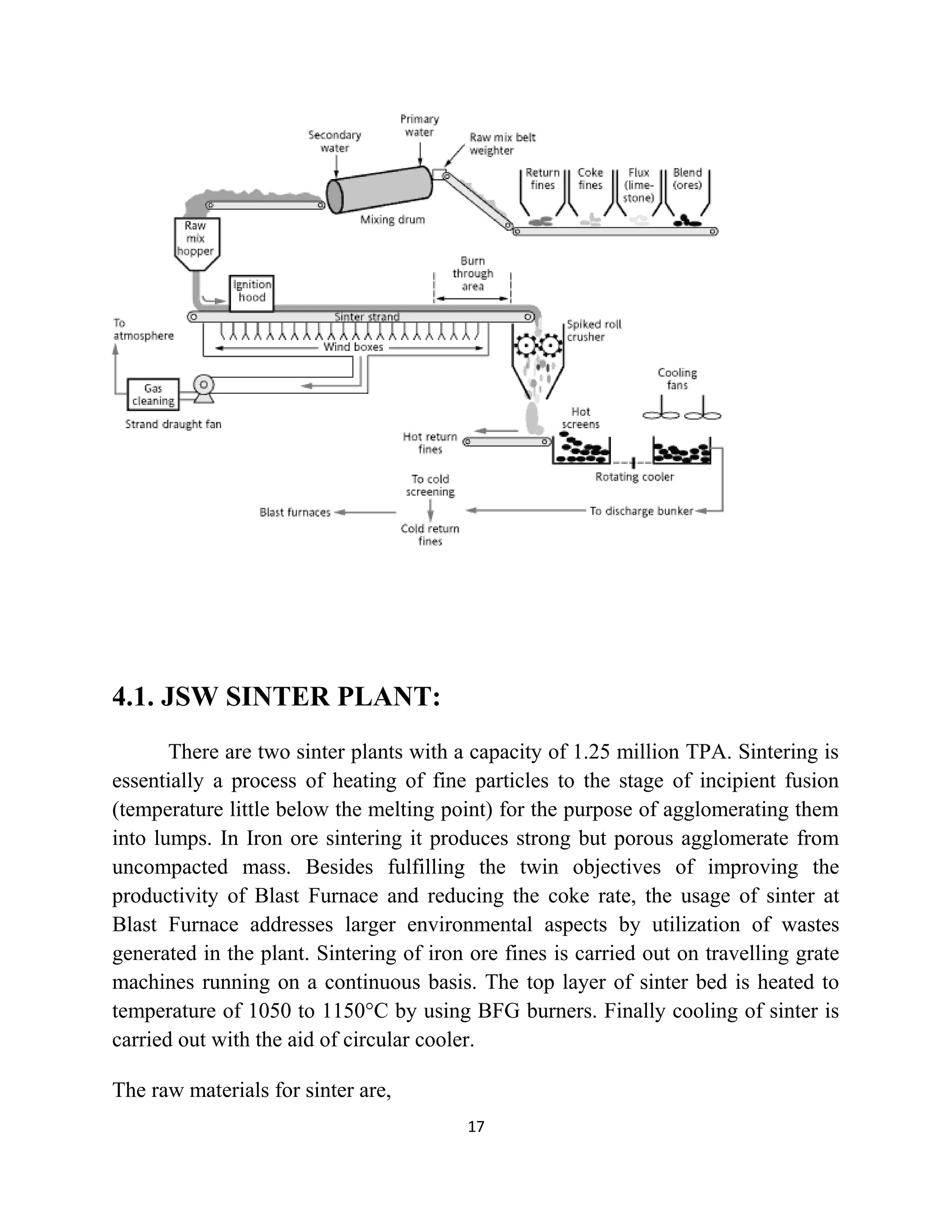
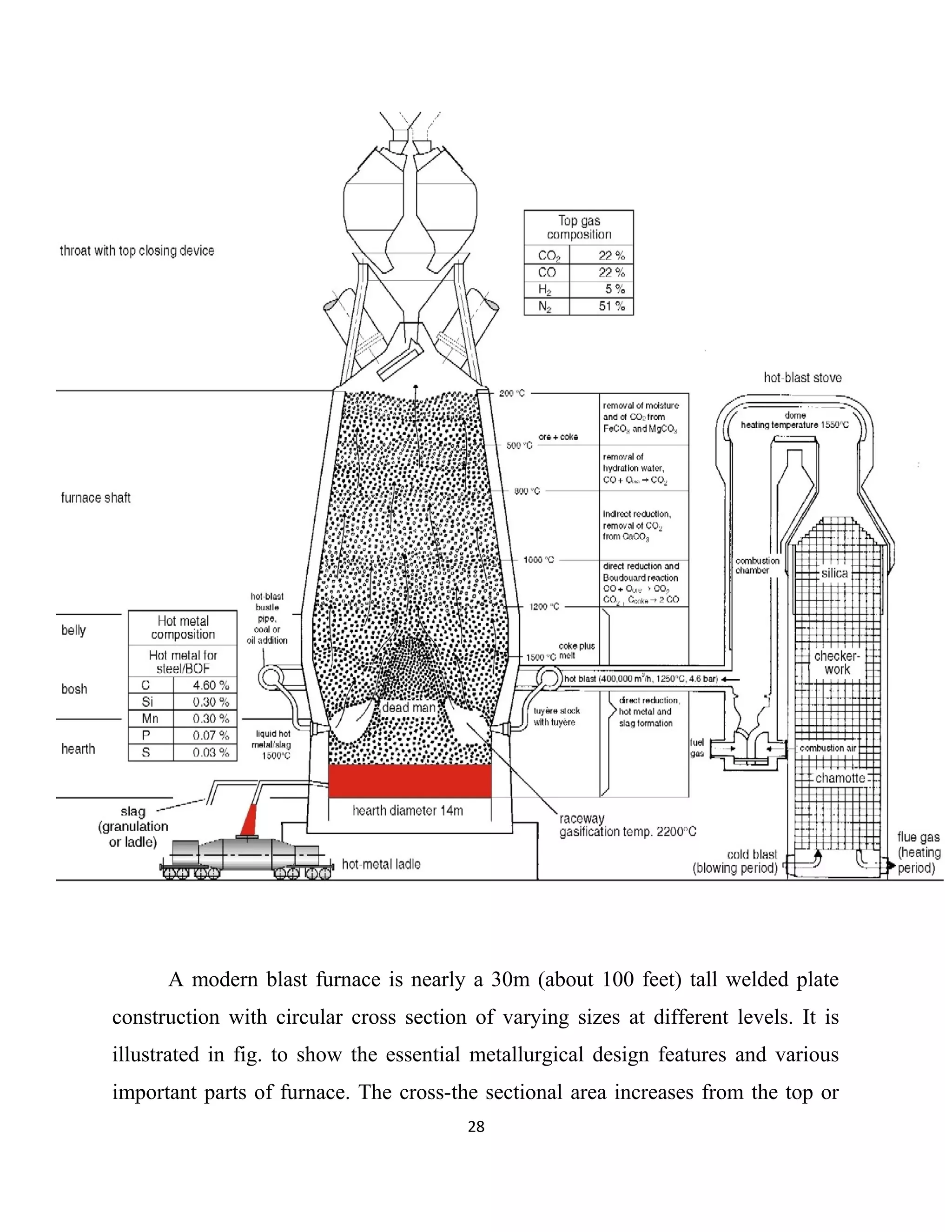
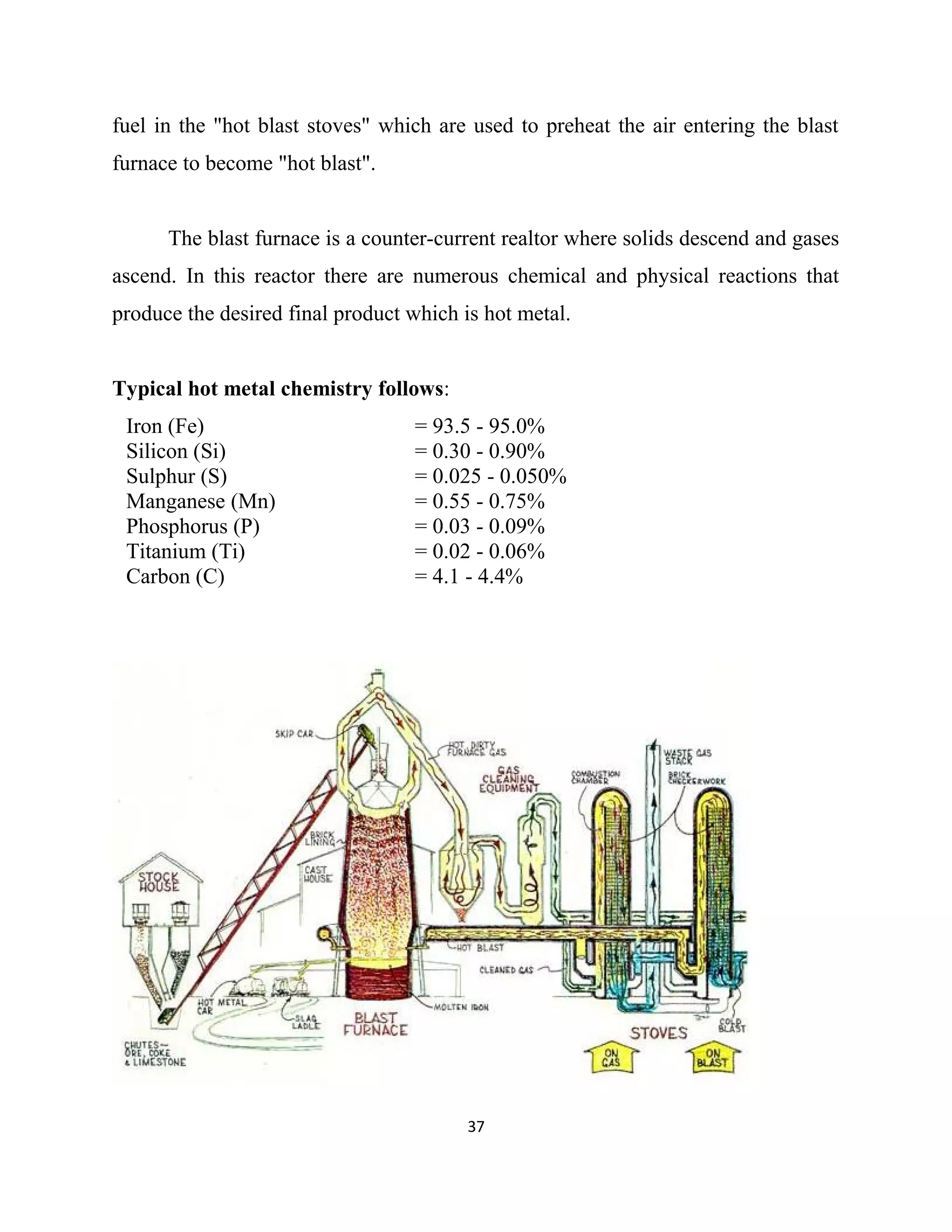
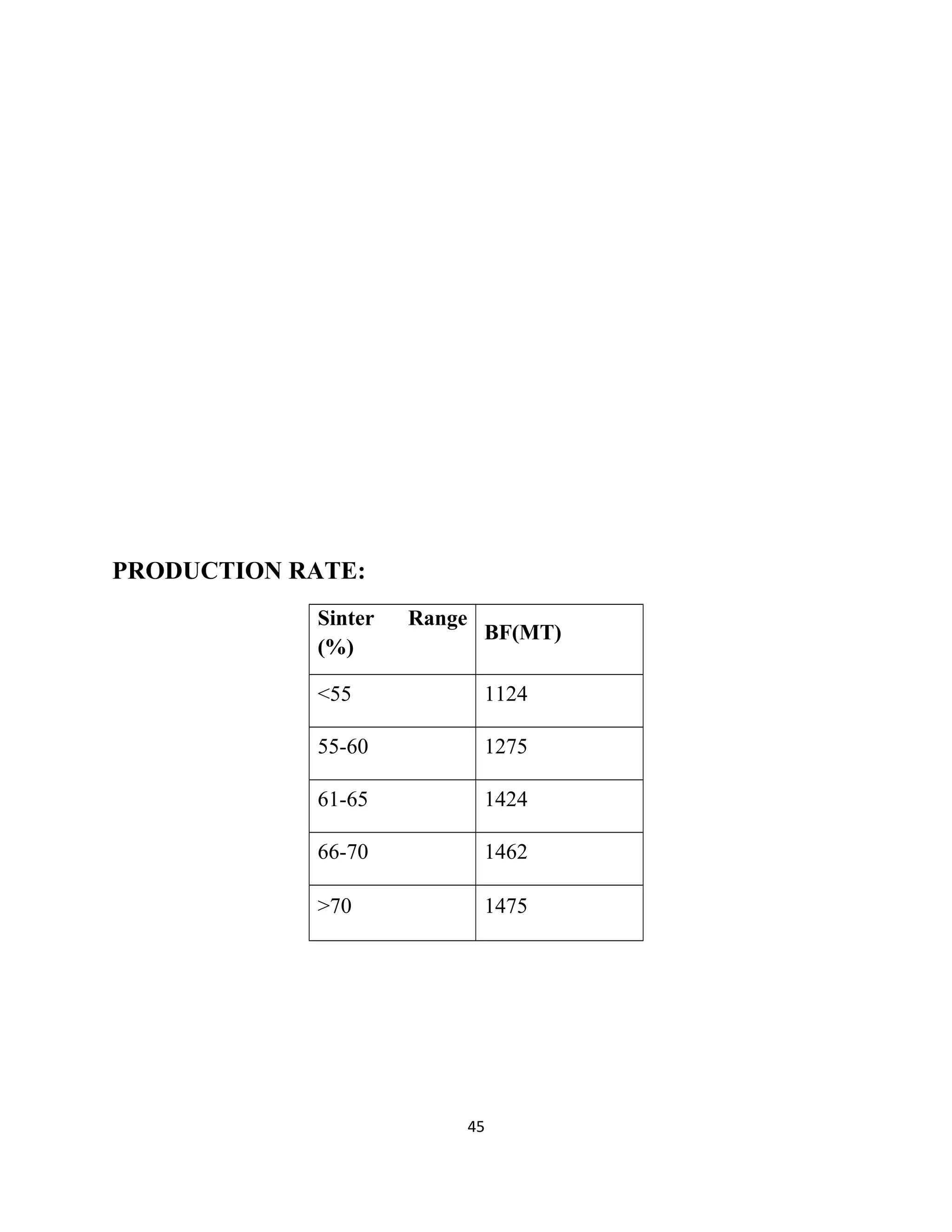
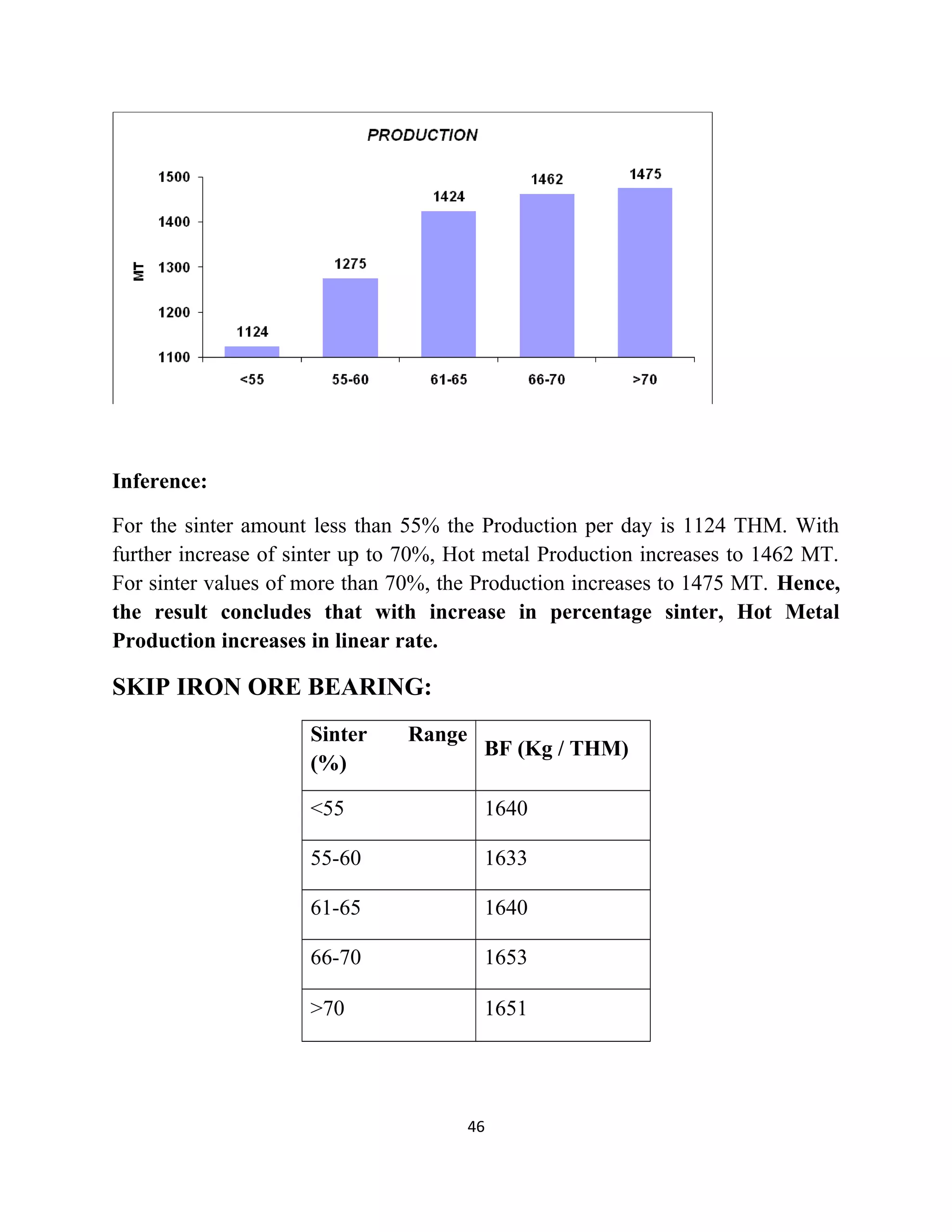
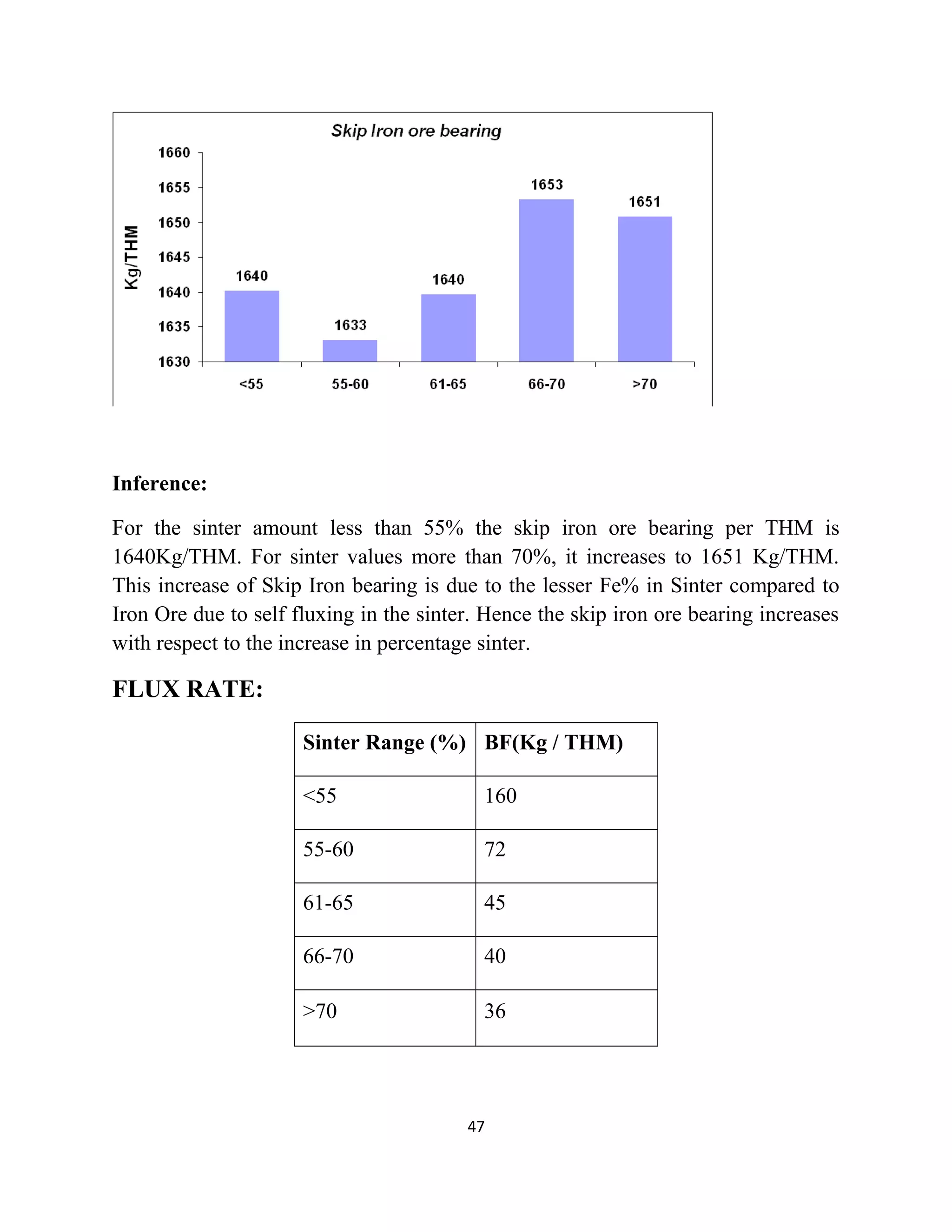
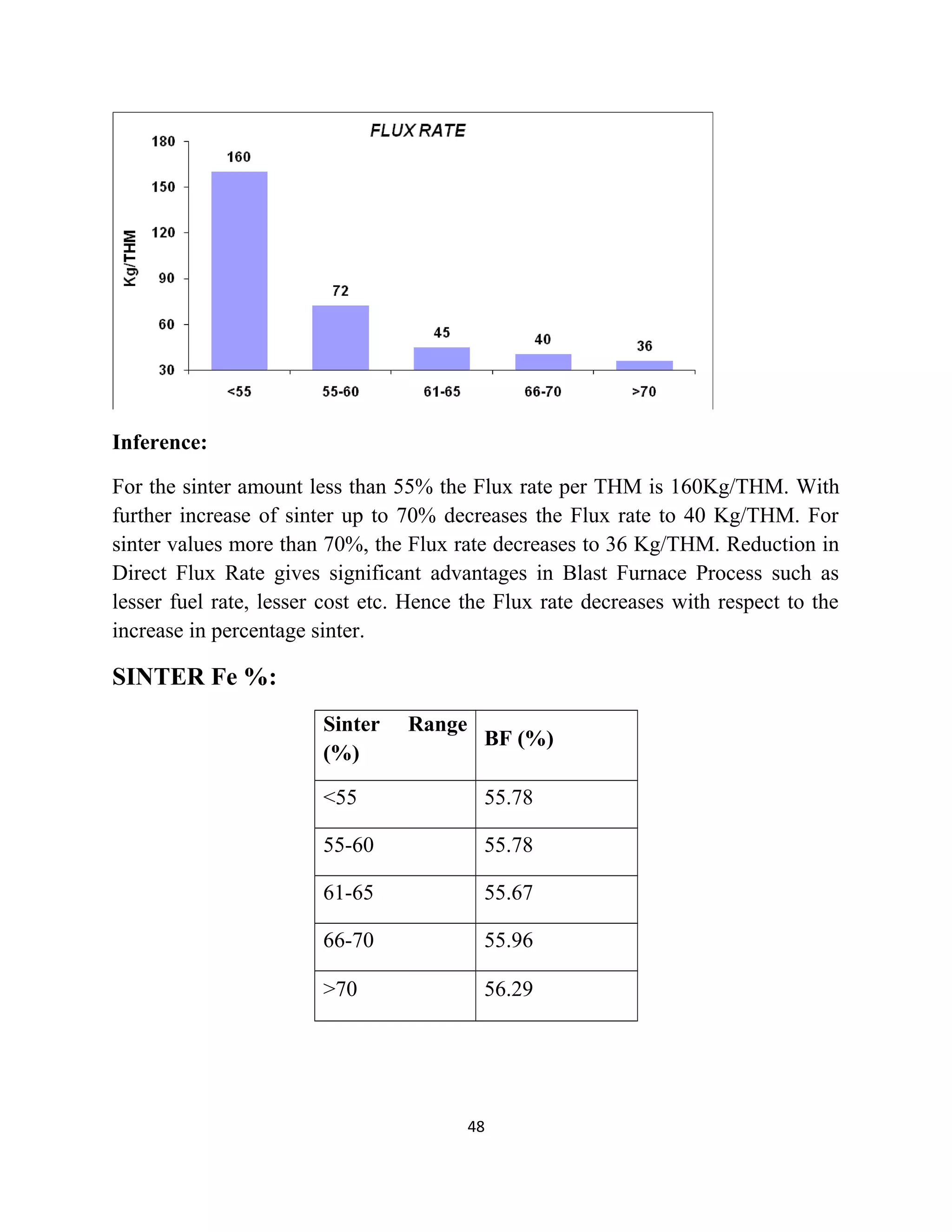
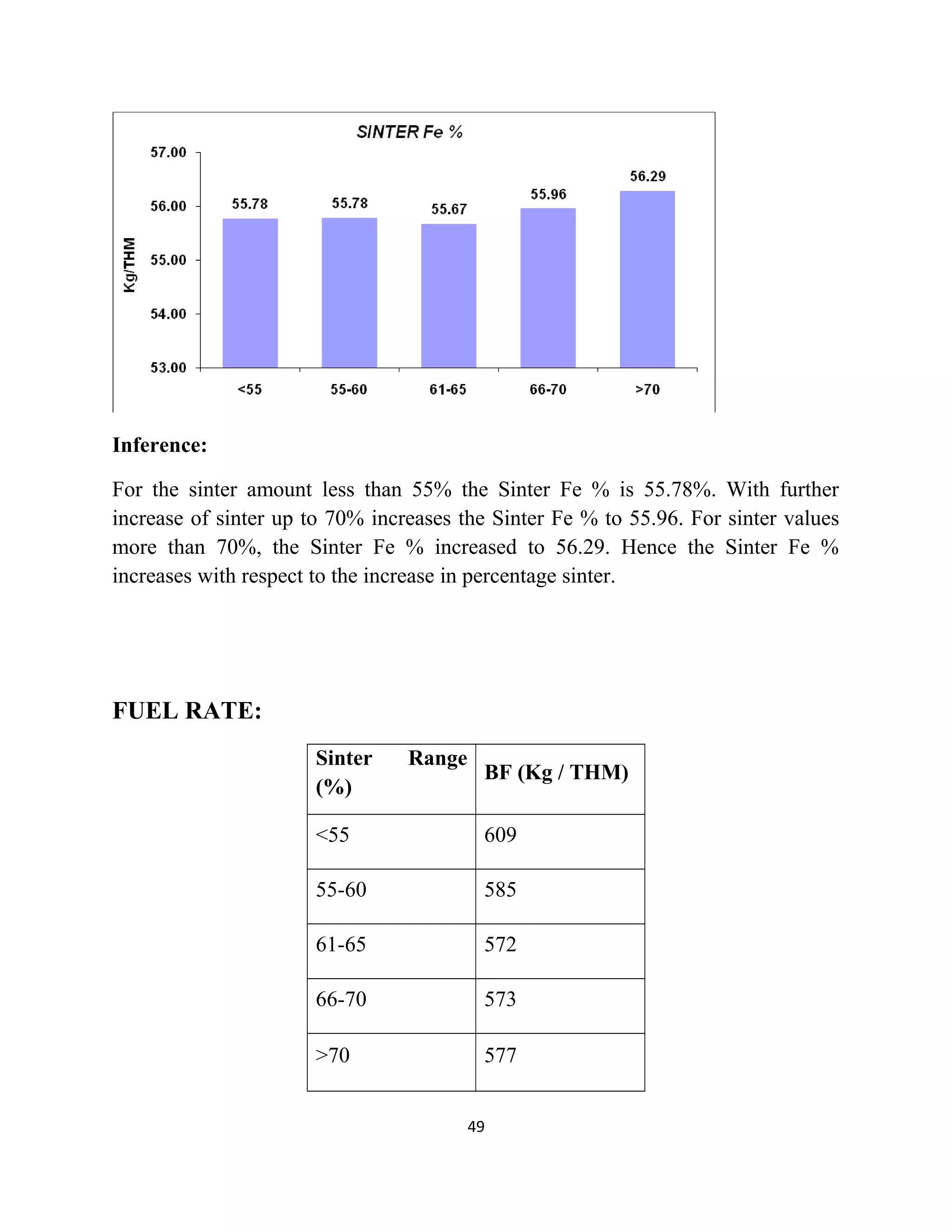
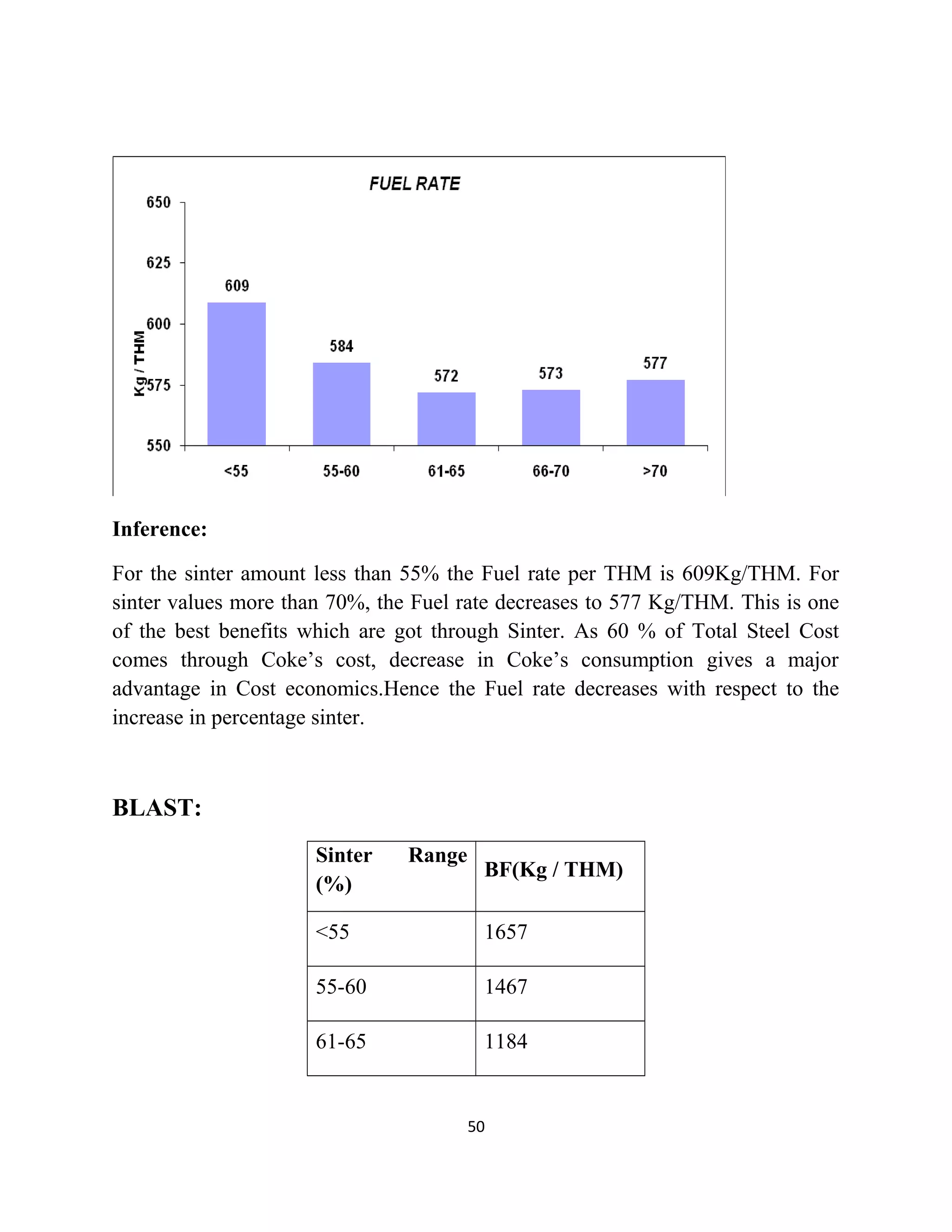
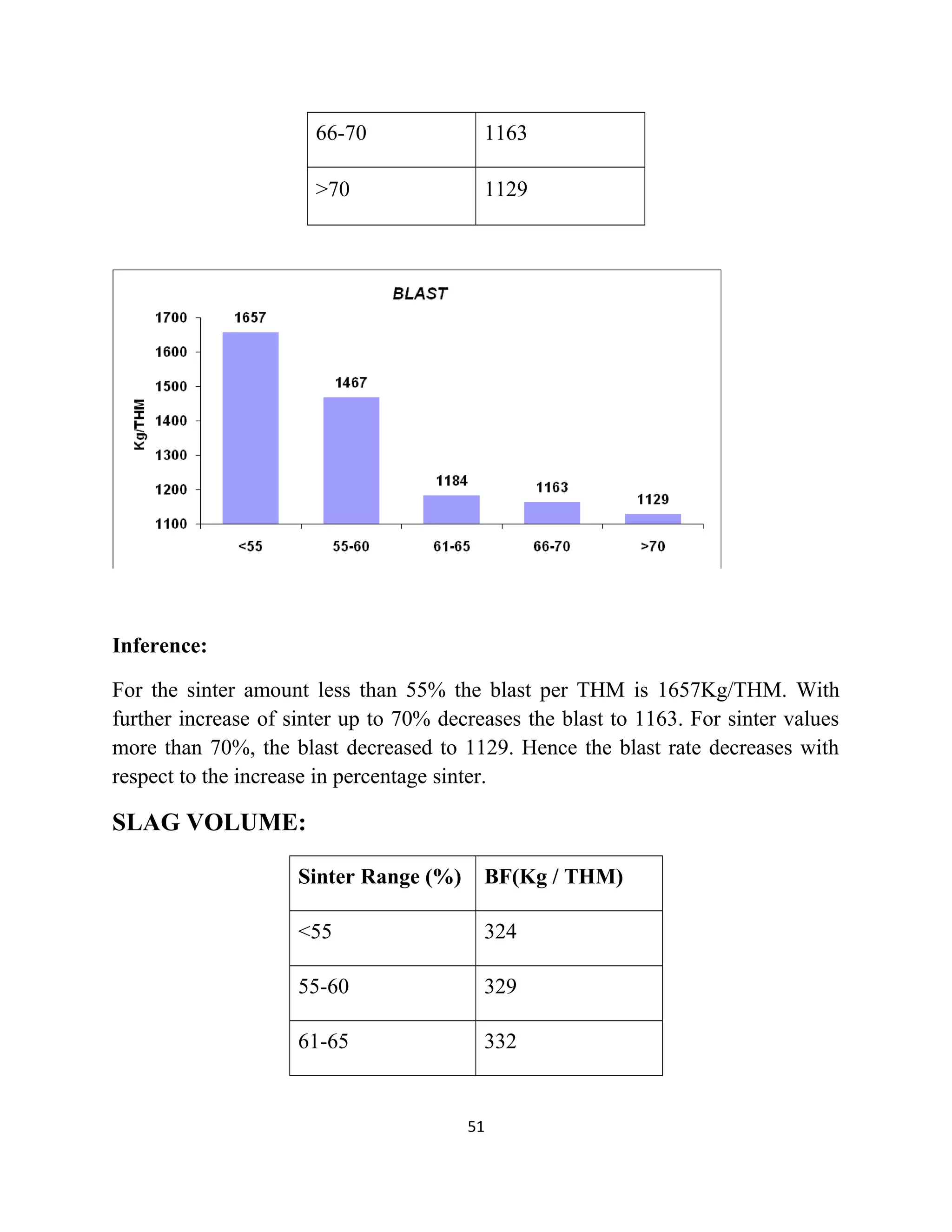
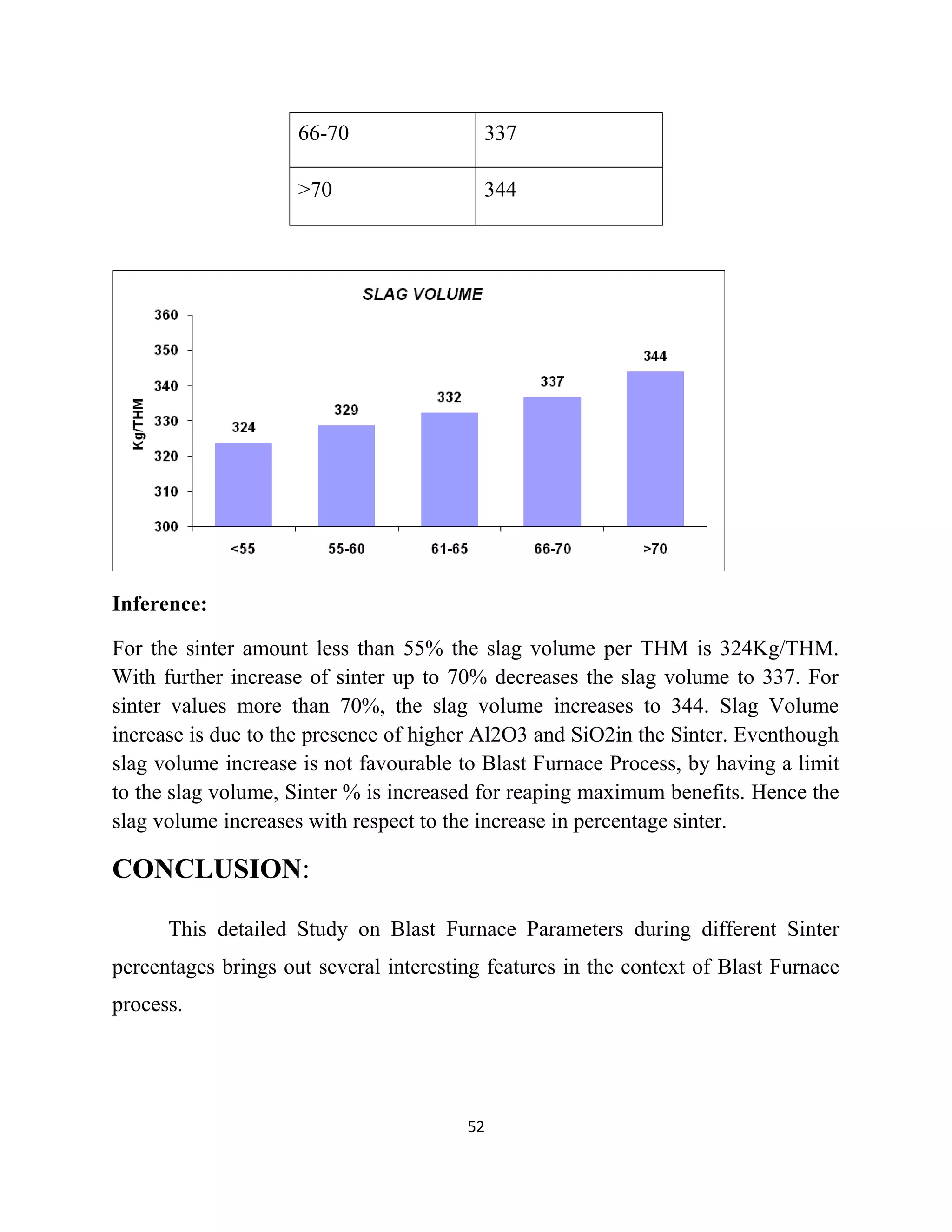
The document details a project by students from the Government College of Engineering, Salem, analyzing the impact of varying sinter percentages on blast furnace parameters at JSW Steel. It discusses the importance of increasing sinter usage to enhance productivity while reducing energy consumption and iron ore usage. Additionally, the paper outlines the various operations and technologies employed at the JSW Salem works steel plant, which focus on environmental sustainability and efficiency in steel production.