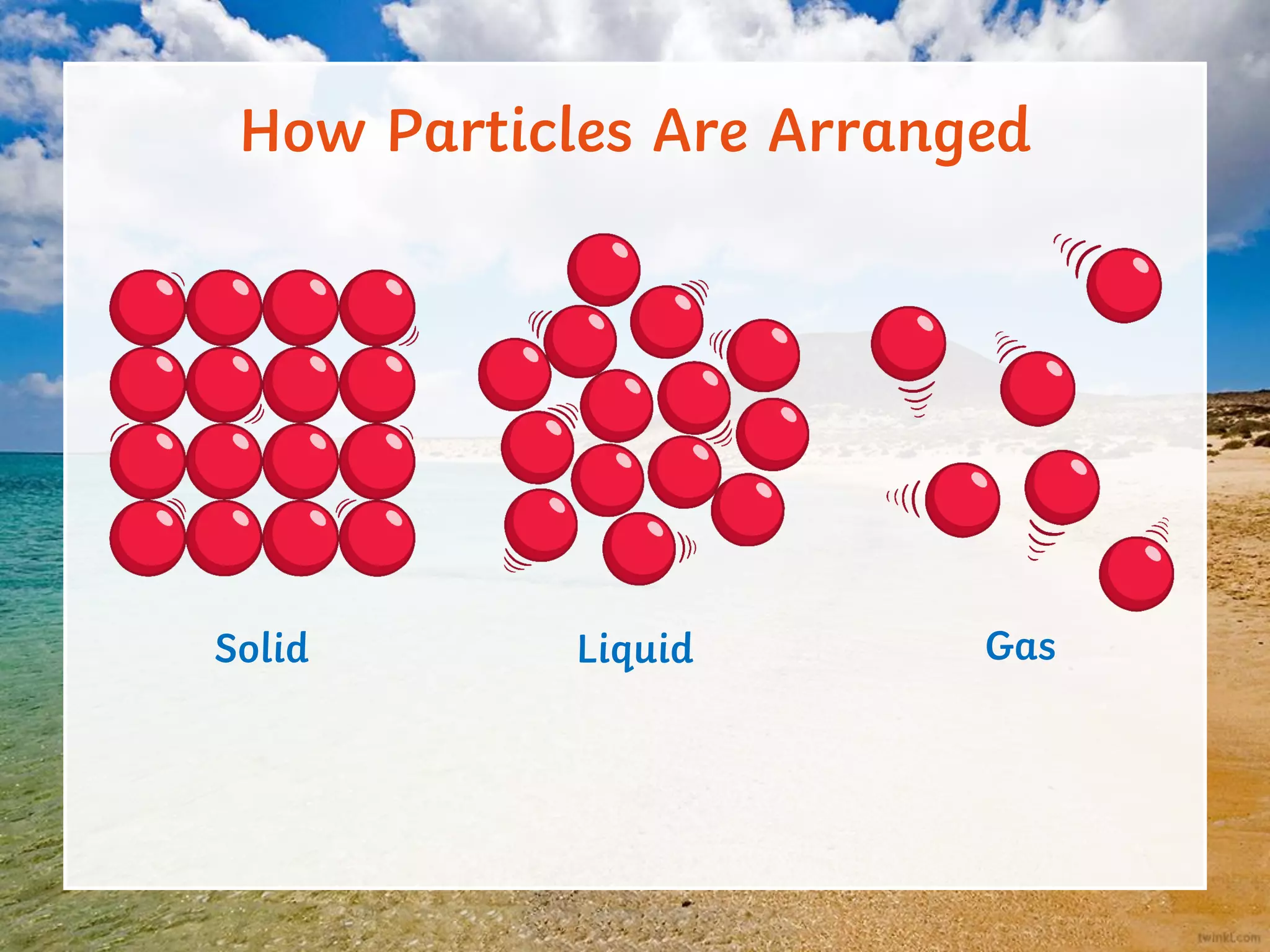
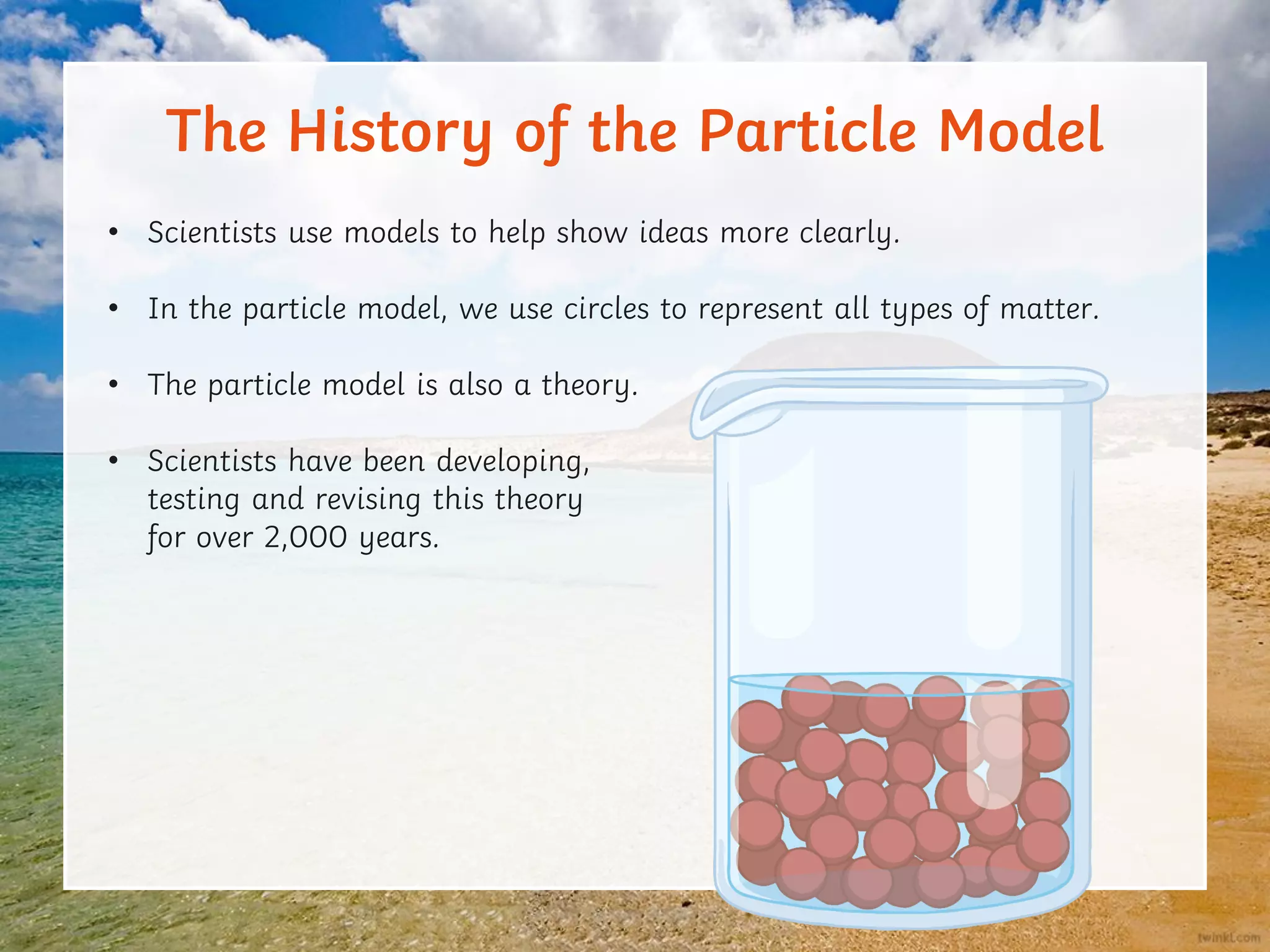
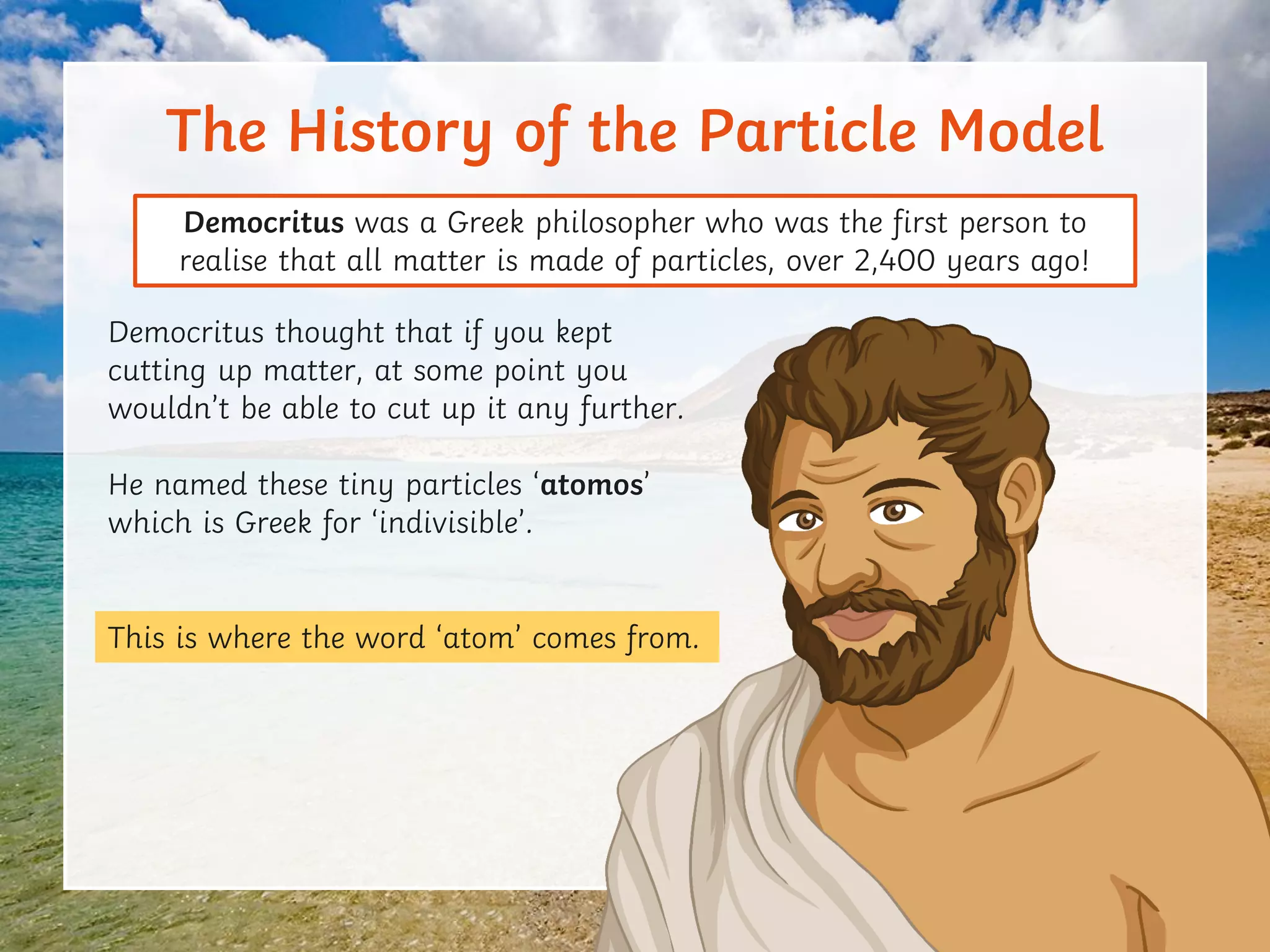
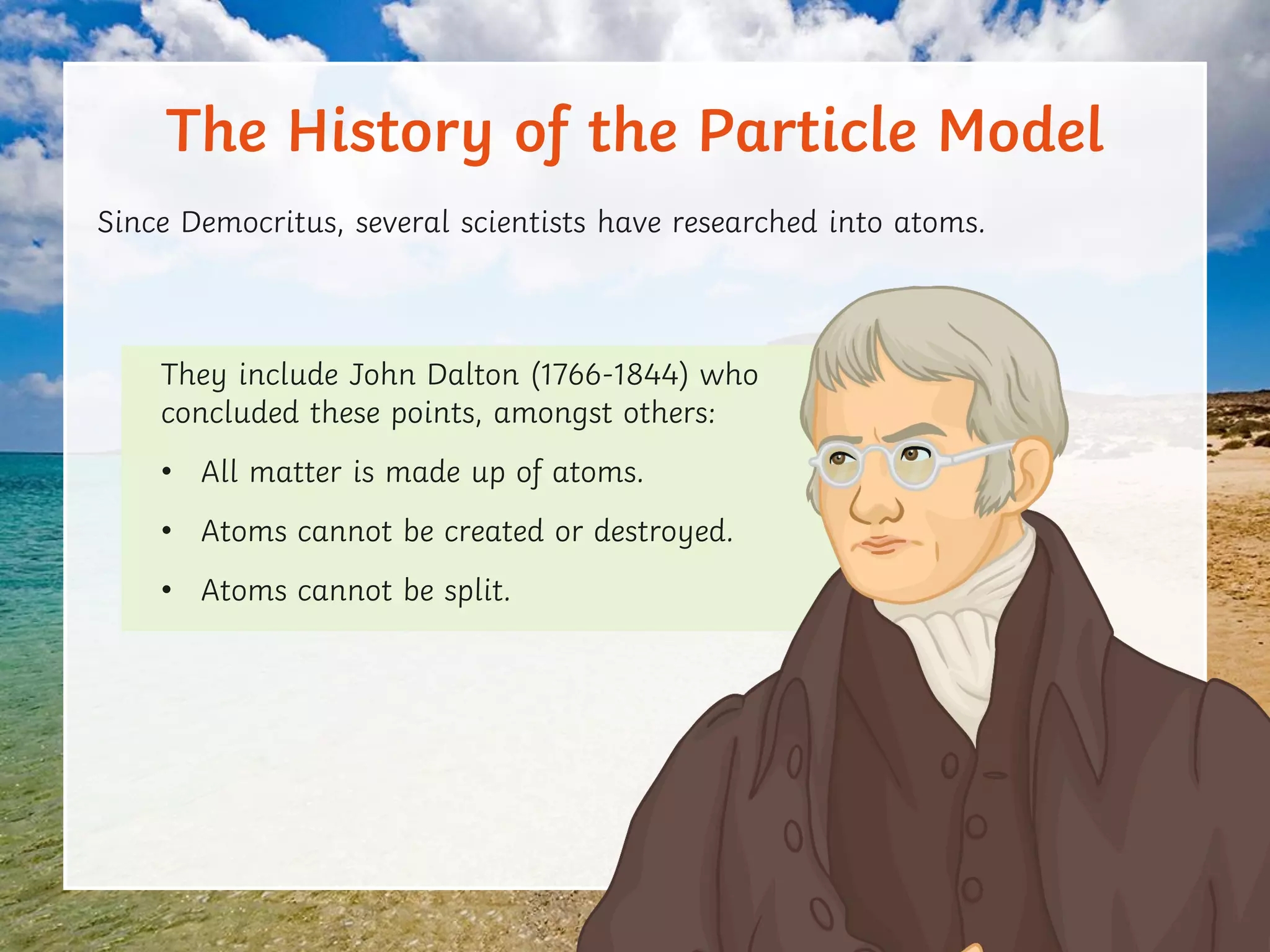
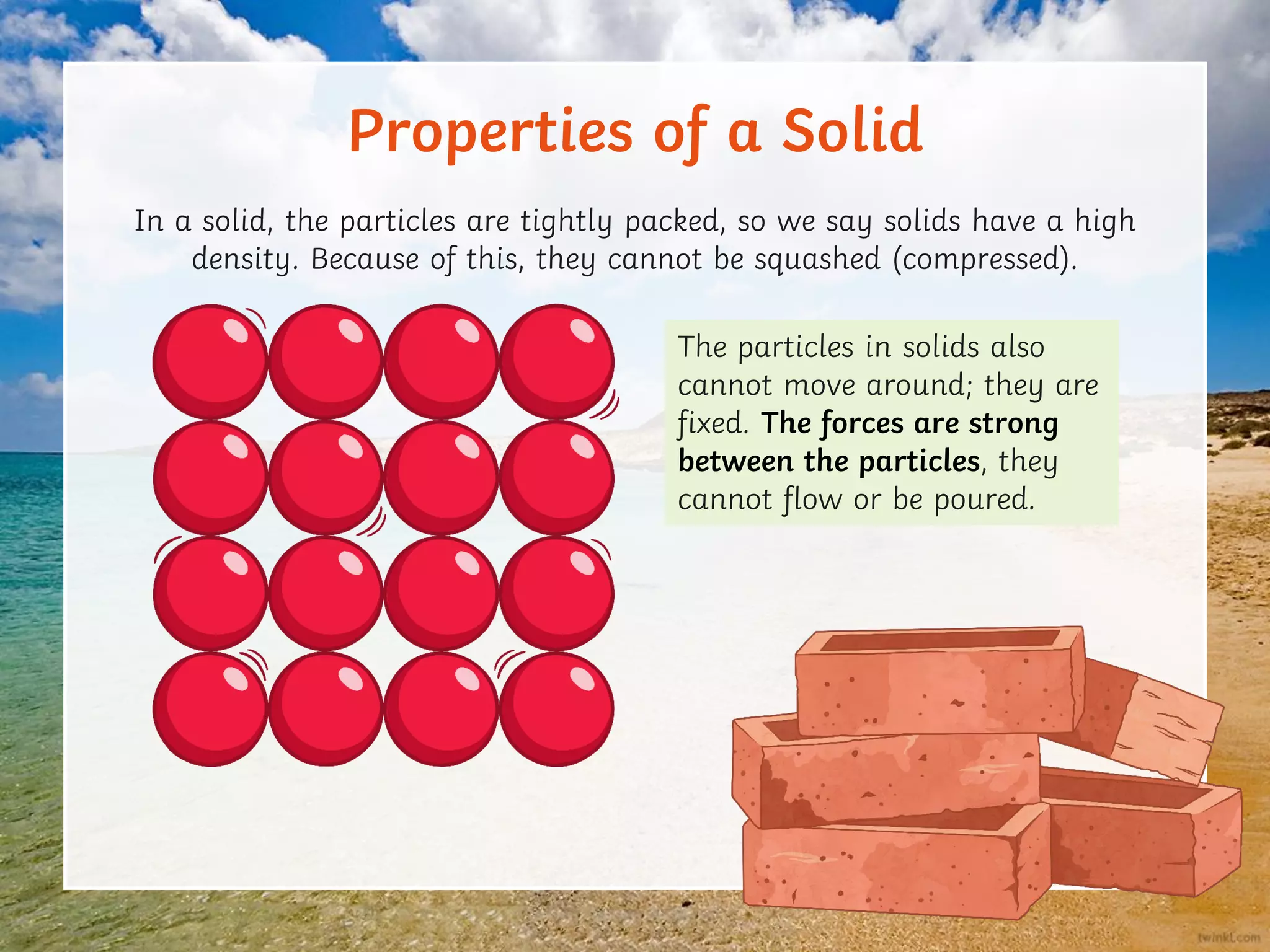
This document discusses the particle model of matter and the key differences between solids, liquids, and gases at a molecular level. It defines density as the mass of a substance per unit volume and explains how density is higher in solids and lower in gases, with liquids being intermediate. The document also outlines how the arrangement of particles in each state of matter determines properties like compressibility, ability to flow, and strength of molecular forces.