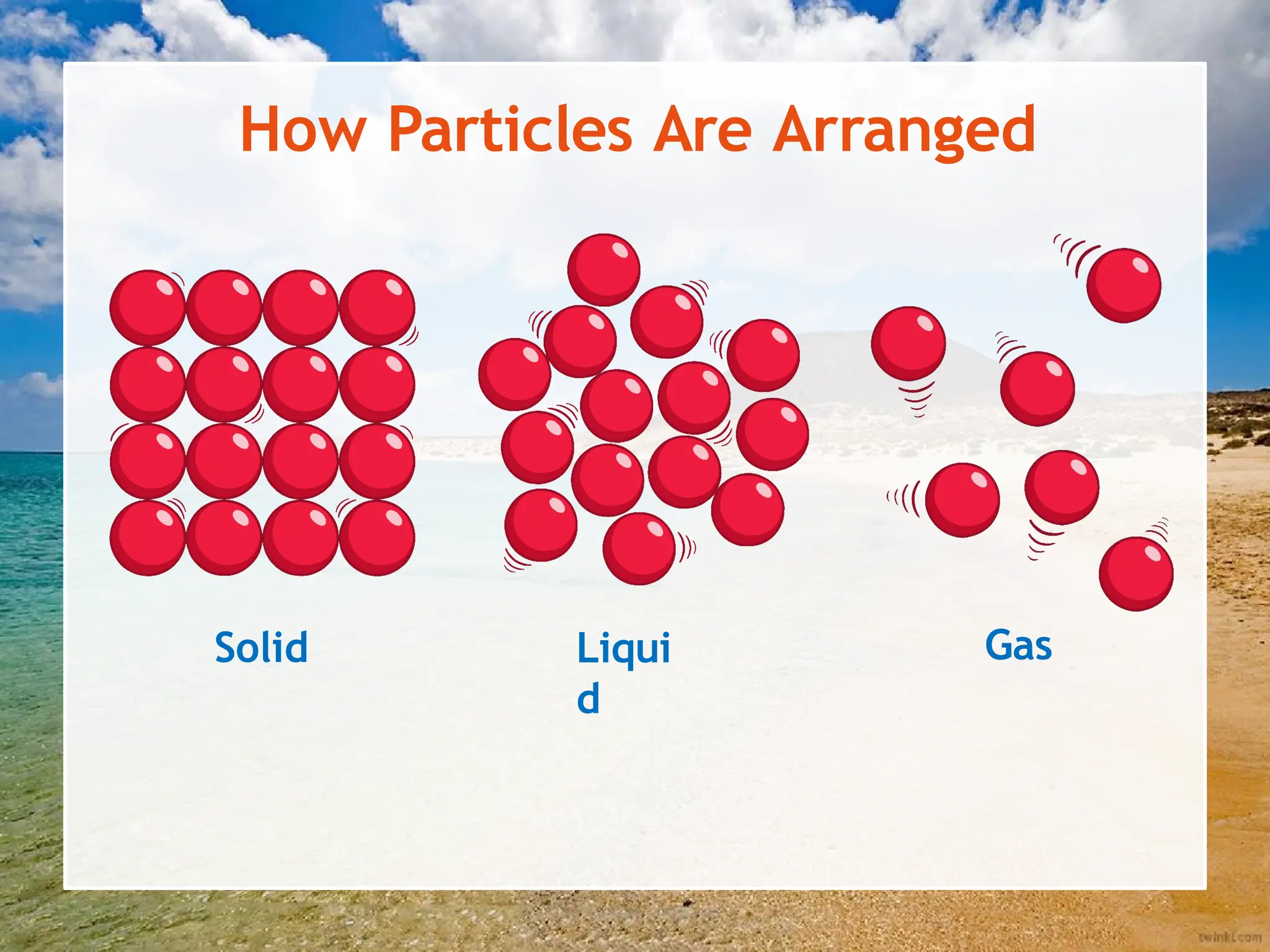
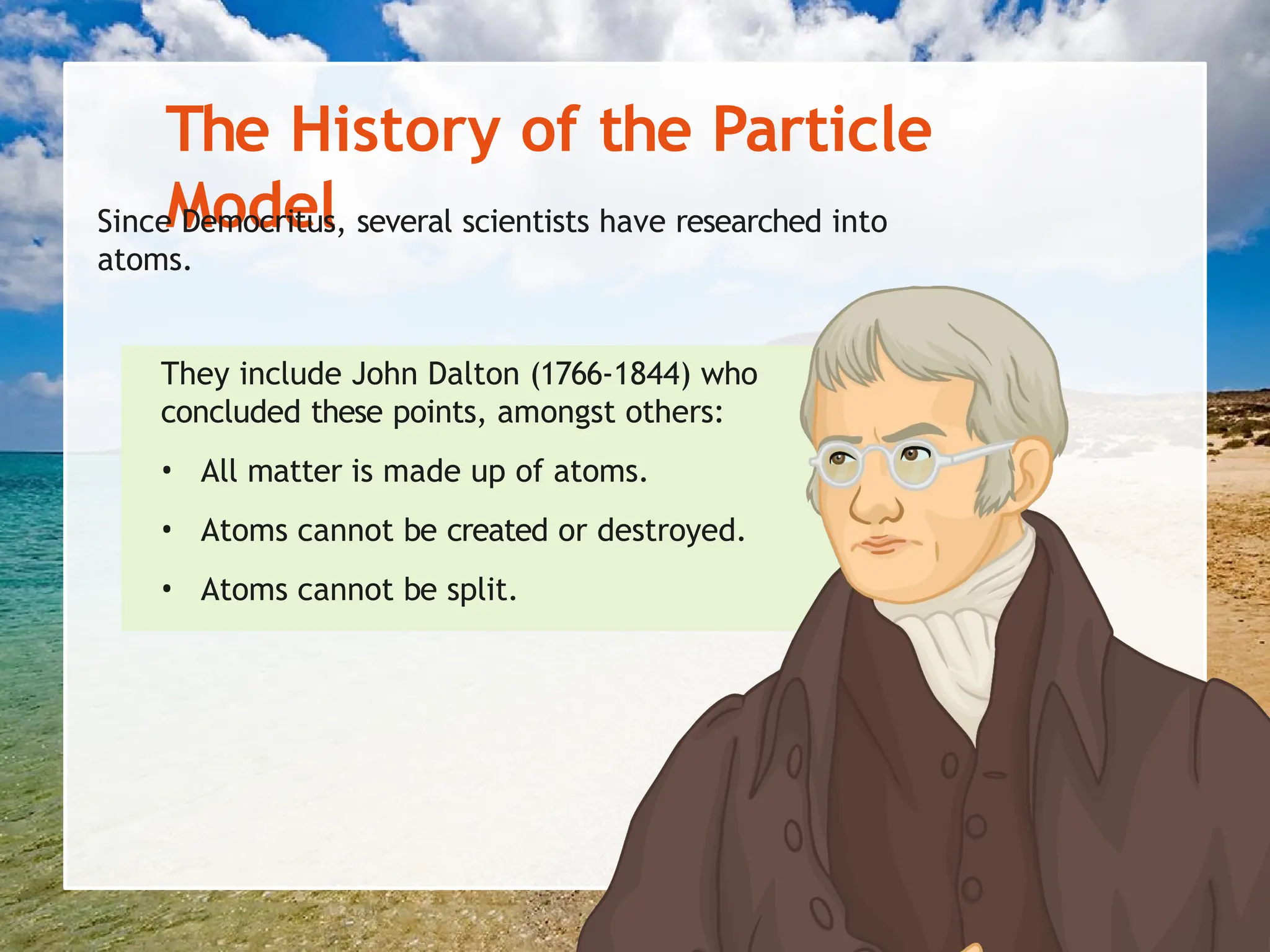
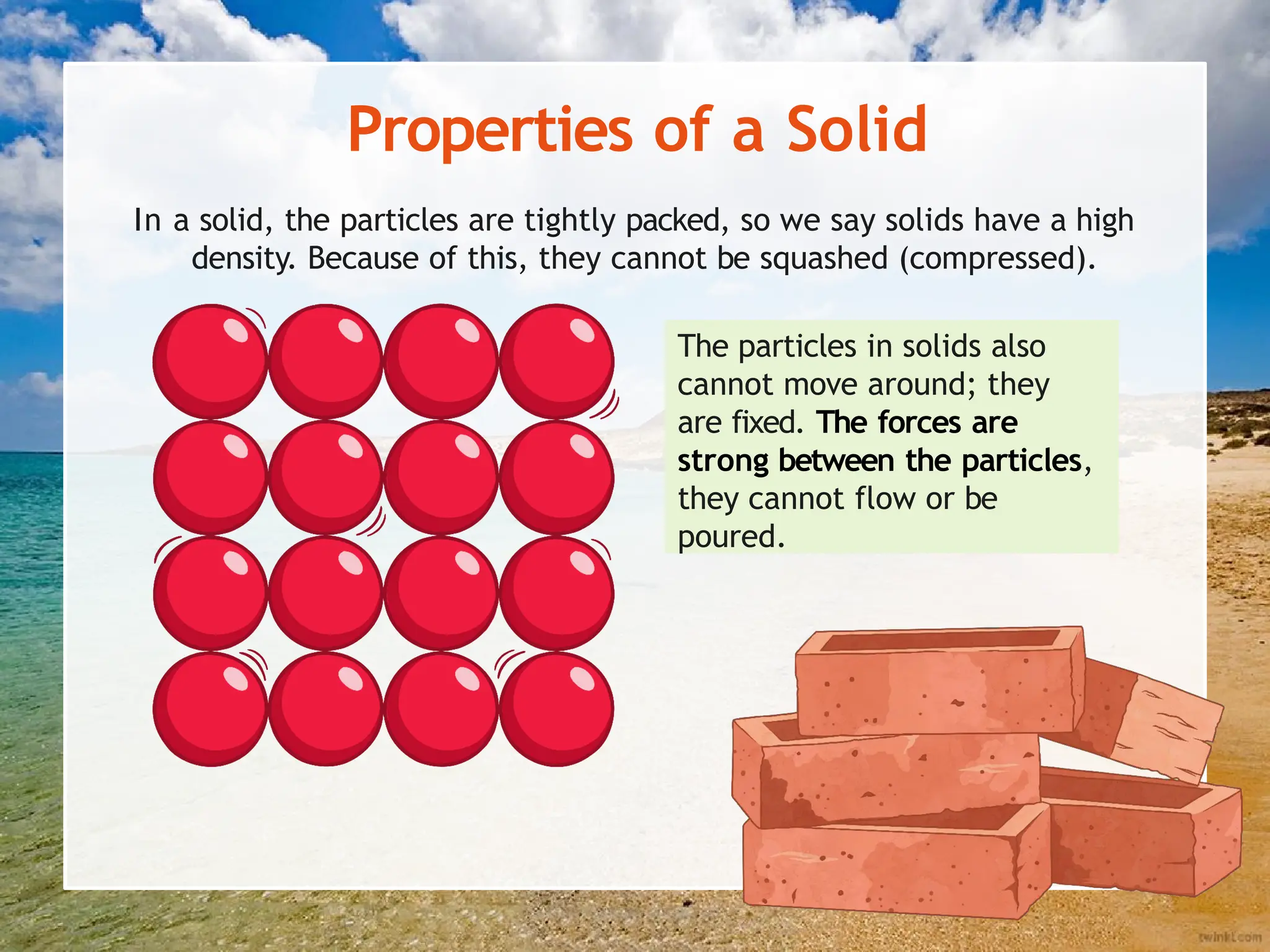
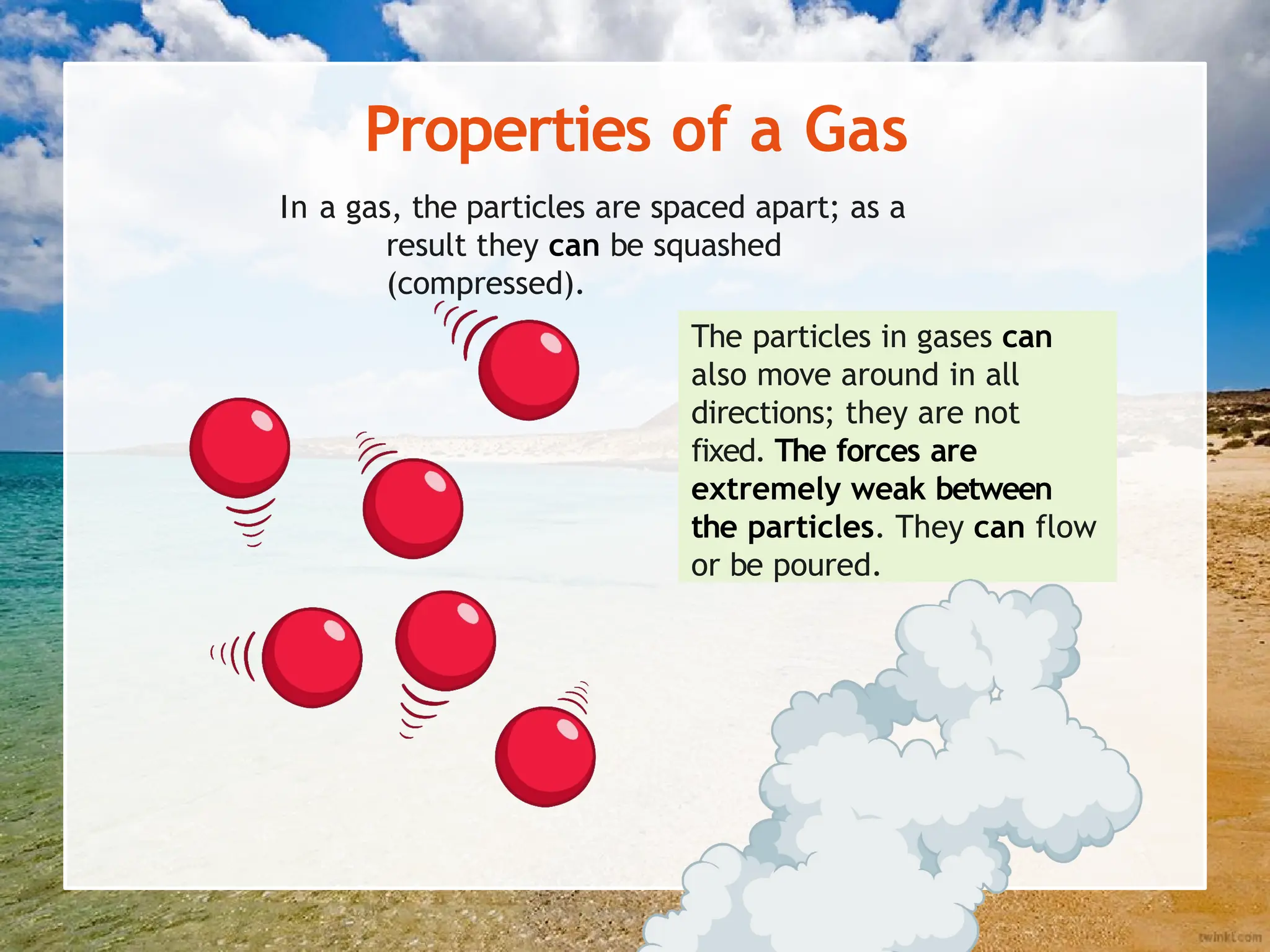
The document explains the arrangement of particles in the three states of matter: solids, liquids, and gases, linking these arrangements to their respective properties. It discusses the history of the particle model, highlighting contributions from philosophers like Democritus and scientists like John Dalton. Additionally, it defines density and illustrates how it varies across different states of matter, with a focus on practical activities to reinforce understanding.