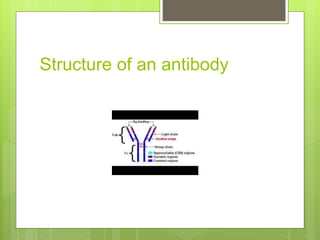
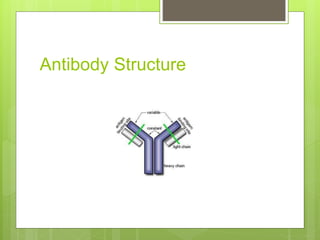
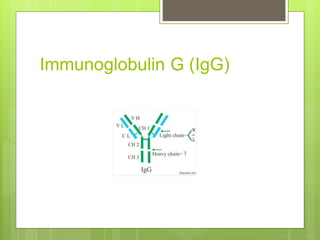
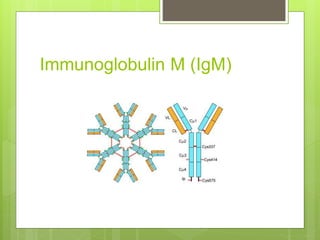
The document discusses the structure and classes of antibodies (immunoglobulins). It notes that antibodies have a Y-shaped structure consisting of two heavy chains and two light chains. The heavy chains have four or five constant domains, while the light chains have two domains. There are five classes of antibodies - IgM, IgG, IgA, IgD, and IgE - which differ in structure and function. IgG is the most abundant antibody and can cross the placenta, while IgM is the first antibody produced against new pathogens. IgA is found in secretions and protects mucosal surfaces.