Heavy metal oxide glasses doped with rare earth ions were investigated for their optoelectronic properties. TeO2–PbO–WO3 glasses were doped with Er3+ and Tm3+ ions and prepared using a conventional melting and quenching method. The glasses exhibited infrared luminescence from the Er3+ and Tm3+ ions at room and liquid helium temperatures due to energy transfer processes between the glass host and rare earth ions. The glasses also showed potential for excitation by Nd-YAG lasers and could enable optoelectronic device applications due to their properties.
![Heavy metal oxide glasses doped by rare earth ions
for optoelectronics
Dominik Dorosz
Białystok University of Technology
Wiejska Street 45D, 15-359 Bialystok, Poland
Jan Wasylak
AGH University of Science and Technology, Faculty of Materials Science and Ceramics,
Ave. Mickiewicza 30, 30-059 Kraków, Poland
ABSTRACT
Heavy metal oxide glasses in the system TeO2–PbO–WO3 doped by selected rare earth ions have been presented. The
method of their preparation and physical properties were determined.
Infrared luminescence of Er3+
and Tm3+
ions embedded in TeO2–PbO–WO3 glass hosts is reported for room and helium
liquid temperatures. The substantial influence of energy transfer processes between the glass host and Er3+
and Tm3+
doped ions is shown experimentally through the dependences of photoluminescence on light polarization and excitation
wavelength. The presented spectra of the rare earth doped glasses indicate on a possibility of their excitation by the Nd-
YAG laser with wavelength about 1,06 µm. Because of their properties this kind of glasses can be used for construction
of optoelectronic devices.
Keywords: IR glasses, rare earth, optoelectronics.
1. INTRODUCTION
Telluride glasses as distinct from the silicate, boron, phosphorus or germanium glasses do not show the glass forming
properties when they are melted separately. Sufficiently stable glasses can be obtained in the system BaO–TeO2
containing 11-18 wt% of BaO. It is obvious that TeO2 can behave as a glass forming oxide, but this takes place only in
strictly defined conditions. Glasses of TeO2 type are thus numbered as belonging to the group of oxide glasses “forming
the glassy state on condition”. This group comprises also the oxides such as: SeO2, MoO3, WO3, Bi2O3.
In the year 1962 Imaoka carried out extensive investigations of the glass forming ability of the systems of two-
component telluride glasses [1]. Among them it can be found also the combinations of tellurium with the heavy metal
elements. They are slightly melted at temperatures below 1000o
C. They possess relatively high refractive index (higher
than 2.0), which is a consequence of high polarizability of tellurium ions. Due to the same reasons possess they also large
third order non-linear optical susceptibilities which are even 100 times higher than in the case of the traditional silicate
glasses [2].
Recently one can see an increasing interest to glasses which are transparent in mid-IR region [1,3-6]. This one is caused
by a possibility of their using as new promising materials for optical fibers and IR waveguides. We have shown [2] that
doping of the glasses by Er3+
and Tm3+
ions may present a new way for creating of quasi-continuous IR lasers. In the
present work we will try to verify a possibility of using tellurium-containing glasses which are doped simultaneously by
Er3+
and Tm3+
in moderate weighting ratio (about 1-1.5 %). Besides they possess high transparency in the IR spectral
range and low dispersion. They might be of interest as materials for optical fibres, optical modulators, parametrical
amplifiers etc.
Particular interest present rare earth doped materials. This is caused by their relatively large radius compared to the other
compounds as well due to specific luminescent properties of the rare earth ions. In the present work we present the data
concerning the influence of the rare earth on the emission properties of glasses under the investigation.
Invited Paper
Optical Fibers: Technology, edited by Jan Rayss, Brian Culshaw, Anna G. Mignani,
Proc. of SPIE Vol. 5951, 595106, (2005) · 0277-786X/05/$15 · doi: 10.1117/12.622198
Proc. of SPIE Vol. 5951 595106-1
Downloaded From: http://proceedings.spiedigitallibrary.org/ on 05/28/2015 Terms of Use: http://spiedl.org/terms](https://image.slidesharecdn.com/10-151016121020-lva1-app6891/85/10-1117-12-622198-1-320.jpg)
![2. EXPERIMENTAL METHOD
TeO2–PbO–WO3 glasses used in this work were synthesized by a conventional melting and quenching method. Pure
oxide materials (99.996%) were used to prepare glass batches (20g) in the investigated system doped by Er2O3 and
Tm2O3 (99.996%). The glasses were melted in covered gold-platinum crucibles, in an electric furnace, at the temperature
800°C. The melting time was 30 min. The melted mass of glass was poured into a brass mould preheated around the
glass transition temperature and annealed in the temperature range 330-370°C depending on the glass composition. The
glassy state was defined visually after pouring the molten glass in a brass mould. The amorphous state was tested by the
method of X-ray diffraction on a roentgen meter Seifert – FMP XRD7. Thermal properties were measured on (2g)
samples from powdered glass in corundum crucibles by means of derivatograph Q - 1500D. The heating rate was
10°/min. The density of the glass was determined by the method of hydrostatic weighing. Light transmission
measurement of samples, having the form of polished plates 20×10×1 mm was carried out in the range 0.2 – 1.1 µm on a
spectrophotometer SPECORD UV-VIS Carl Zeiss Jena and in the range 2.5 – 25 µm on a spectrophotometer SPECORD
M80 Carl Zeiss Jena. On the basis of the transmission measurements of the glasses their refractive indices n(λ) were
calculated [7].
The investigations were carried out by the classical spectroscopy methods of the transfer of luminous fluxes through the
sample [8]. The exciting source were various pulse lasers: Xell of the power 0,8 W and wave length 0, 714 µm , Kr II
(0,94 W) – λ = 0,66 µm, Ar II (1.1 W) - λ =0.528 µm and the nitrogen laser (0.8 W). Simultaneously the induced
luminescence in infrared was examined, excited by the photoinductive lasers: YAG – Nd ( W = 30 MW, τ = 30 ps, λ =
1.06 µm ) and HF of the power 1.2 W and the wave lengths 2.64, 2.87 and 3.26 µm. The duration of the laser pulses was
within the limit 2 –8 ns, and their energy was 200- 700 µJ. The physical properties obtained glasses were examined.
Table 1. Chemical compositions.
Glass Chemical compositions
A 10PbO- 30WO3- 60TeO2 – Er3+
+Tm3+
=0,5 %wag. Er3+
: Tm3+
= 1: 1
B 10PbO- 30WO3- 60TeO2 – Er3+
+Tm3+
=0,5 %wag. Er3+
: Tm3+
= 2: 1
C 10PbO- 30WO3- 60TeO2 – Er3+
+Tm3+
=0,5 %wag. Er3+
: Tm3+
= 3: 1
D 10PbO- 30WO3- 60TeO2 – Er3+
+Tm3+
=0,5 %wag. Er3+
: Tm3+
= 1: 2
E 10PbO- 30WO3- 60TeO2 – Er3+
+Tm3+
=0,5 %wag. Er3+
: Tm3+
= 1: 3
F 8PbO- 2PbCl2- 30WO3 - 60TeO2
G 5PbO- 5PbCl2- 30WO3 - 60TeO2
H 3PbO- 7PbCl2- 30WO3 - 60TeO2
I 10PbO- 10WO3- 80TeO2
J 10PbO- 20WO3- 70TeO2
K 10PbO- 30WO3- 60TeO2
L 10PbO- 40WO3- 50TeO2
3. RESULTS
A typical DTA curve for TeO2–PbO (PbCl2)–WO3 glass doped with 0.5 wt% Er3+
+Tm3+
is shown in Fig. 1. The Tg,Tx
and Tm indicate the glass transition, crystallization and melting temperatures, respectively. The thermal stability of the
glass is defined by ∆T= Tx -Tg. The thermal stability of glass is essential with regard on necessity the glass recasting in
process of optical fibre drawing. Crystalline interpolations in processed core glass cause scattering losses, enlarging
attenuation. The obtained glasses have ∆T in the range 51-130. The glasses doped by RE ions poses thermal stability
over 100, so they are stable against crystallisation.
Proc. of SPIE Vol. 5951 595106-2
Downloaded From: http://proceedings.spiedigitallibrary.org/ on 05/28/2015 Terms of Use: http://spiedl.org/terms](https://image.slidesharecdn.com/10-151016121020-lva1-app6891/85/10-1117-12-622198-2-320.jpg)
![383
300 400 500 600
Temperature C)
In order to determine the structure of the glasses in the examined PbO-WO3-TeO2 system there have been prepared
infrared spectra from a section of the glassy state area at steady content of lead oxide 10% mol (Fig.2).
Fig. 1. A typical DTA curve the TeO2–PbO (PbCl2)–WO3 glass.
Fig.2. Middle (MIR) and far (FIR) IR spectra of glasses from the system PbO-WO3-TeO2: 1 – 10PbO-10WO3-80TeO2; 2 – 10PbO-
20WO3-70TeO2; 3 – 10PbO-30WO3-60TeO2.
The registered infrared spectra show the presence of two absorption bands situated at the frequency of about
920-930 cm-1
and 850-860 cm-1
, which correspond to the vibrations of tungsten ions exist in the forms of [WO4] and
[WO6] respectively. The bands of at 770 - 780 cm-1
and 640- 650 cm-1
are attributed to the vibration of stretching
tellurium and oxygen bond (Te-O) in TeO3 trigonal pyramids and TeO4 trigonal bipyramids. The bands at 477- 481 cm-1
correspond to the symmetric stretching of the oxygen bridges Te–O–Te. The band at 320 cm-1
, could be attributed to
stretching of the oxygen bridges Te-O-Pb [9]. Analysing the IR spectra, it is possible that replacing TeO2 by WO3 causes
the growth of intensity ratio of TeO3 to TeO4. The increasing TeO3 groups would cause the optical non-linearity to
decrease [10].
Proc. of SPIE Vol. 5951 595106-3
Downloaded From: http://proceedings.spiedigitallibrary.org/ on 05/28/2015 Terms of Use: http://spiedl.org/terms](https://image.slidesharecdn.com/10-151016121020-lva1-app6891/85/10-1117-12-622198-3-320.jpg)

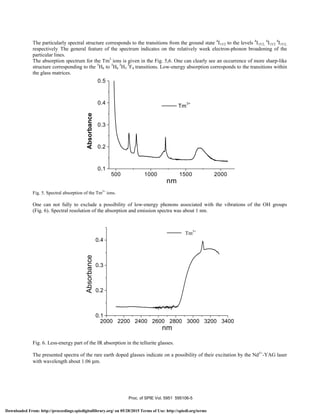
![In the Fig. 7 are given emission spectra at 1530 nm, originating from the transitions between the levels 4
I15/2 to 4
I11/2 of
Er3+
.
1450 1500 1550 1600 1650
0,0
10,0
20,0
30,0
40,0
Er
3+
Emission
λ [nm]
Fig. 7. Emission spectrum of Er3+
in tellurite glasses.
Additional maximum at 1560 nm corresponds to the transitions from 4
I13/2. The broadening of the corresponding levels of
the rare earth’s gives an opportunity to obtain an information concerning the interaction between the rare earth ions and
glass matrix. This confirms a moderate electron-phonon interaction. Substantial shift with respect to the absorption band
at 1690 nm is revealed. This reflects its higher applicability with respect to other materials.
The origin of Tm3+
spectrum (Fig.8) is associated with transitions 3
H4->3
H6, which are very sensitive to the non-radiated
transitions from higher levels 5
H4. The transport between the excited rare earth levels plays here a crucial role.
1650 1700 1750 1800 1850 1900 1950
0,0
5,0
10,0
15,0
20,0
25,0
Tm
3+
Emission
λ [nm]
Fig. 8. Emission spectra of Tm3+
in tellurium contained matrices (weighting content about 1.5 %).
From the presented absorption and emission data one can expect a possibility of creation materials possessing large
quantum efficiency within the large spectral range. So we have explored the emission spectra of the glasses with the
Tm3+
/ Er3+
ratio equal about 1 and weighting content about 1 %.
Proc. of SPIE Vol. 5951 595106-6
Downloaded From: http://proceedings.spiedigitallibrary.org/ on 05/28/2015 Terms of Use: http://spiedl.org/terms](https://image.slidesharecdn.com/10-151016121020-lva1-app6891/85/10-1117-12-622198-6-320.jpg)
![From the Fig. 9 one can see existence of two broad emission spectral maxima around 1550 nm and around 1850 nm.
0
20
40
60
1400 1500 1600 1700 1800 1900 2000
λ [nm]
Luminescence[a.u.]
Fig. 9. IR-emission of the glasses with Tm3+
/ Er3+
≅1 at room temperature (bold line) and liquid helium temperature (thin
line).
Decreasing temperature leads to the enhancement of the 1550 nm Er3+
-originated maximum and to a spectral shift of the
1850 nm Tm maximum. This is a consequence of the inter-level transport between the excited levels. This result may be
a first step for creation of continuous IR emission materials, which are of an importance in the novel technology.
4. CONCLUSIONS
The tellurium-contained glasses possessing simultaneously Er3+
and Tm3+
ions with weighting units about 1 % were
synthesized for the first time. For understanding of the physica insight of the luminescence observed comparison with the
materials possessing separately the ions mentioned is done. We have revealed existence of two broad emission spectral
maxima around 1550 nm and around 1850 nm. Decreasing temperature leads to the enhancement of the 1550 nm Er3+
-
originated maximum and to a spectral shift of the 1850 nm Tm maximum. This is a consequence of the inter-level
transport between the excited levels. This result may be a first step for creation of continuous IR emission materials,
which are of an importance in the novel technology [11,12].
The authors would like to thank prof. I. Kityk for the luminescence measurements.
This work was supported by the State Committee for Scientific Research of Poland –grant No. W/WE/4/04.
5. REFERENCES
1. A.Abd El-Monein. Mater.Chem.Phys., V. 73, (2002), 318.
2. J.Wasylak, K.Ozga, I.V.Kityk, J.Kucharski, Infrared Physics and Technology, V.45, (2004), 253.
3. R.Gopalakrishnan, N.Pajeshwari, S.Buddhudu, Journ.Alloys Comounds, V. 275, (1998), 327.
4. L.G.Hwa, Y.R.Chang, W.C.Chao, Mater.Chem.Phys., V. 85, (2004), 158.
5. V.K.Tikhomirov, D.Furniss, A.B.Seddon, J.A.Savage, P.D.Mason, D.A.Orchard, K.L.Lewis, Infrared Physics and
Technology, V.45, (2004), 115.
6. K.Li, Q.Zhang, G.Zhou, L.Hou, Infrared Physics and Technology, V.43, (2002), 361.
7. Born, M.; Wolf E.: Principles of optics, Pergamon Press, Oxford, 1980.
8. D.R. Simons, A.J. Faber, H. De Waal, Opt. Lett. 20 (1995) 468.
9. S. Khatir, J. Bolka, B. Capoen, S. Turrell, M. Bouazaoui, Journal of Molecular Structure 563-564 (2001) 283-287.
10. J. Lin, W. Huang, Z. Sun, Ch. S. Ray, D. E. Day, Journal of Non-Crystalline Solids 336 (2004) 189–194.
11. J. Dorosz, Ring core fibre as an optical amplifier, Optica Applicata, Vol. XXXIV, No. 4, 2004.
12. T. Pustelny, Physical and Technical Aspects of Optoelectronic Sensors, Publishing House of Silesian Univ. of
Technology, 2005.
Proc. of SPIE Vol. 5951 595106-7
Downloaded From: http://proceedings.spiedigitallibrary.org/ on 05/28/2015 Terms of Use: http://spiedl.org/terms](https://image.slidesharecdn.com/10-151016121020-lva1-app6891/85/10-1117-12-622198-7-320.jpg)