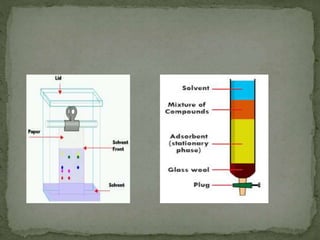
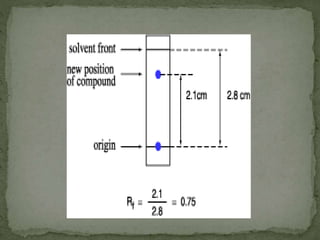
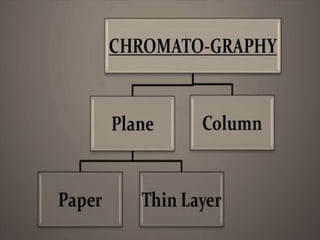
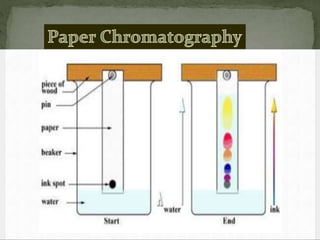
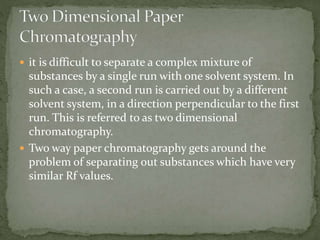
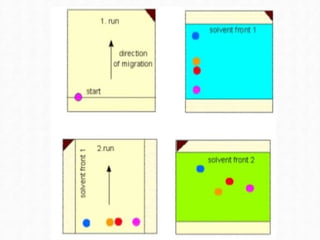
Chromatography is a technique used to separate mixtures by exploiting differences in how components interact with stationary and mobile phases. In paper chromatography, the stationary phase is a strip of paper and the mobile phase is a solvent. As the solvent moves up the paper by capillary action, different components of a sample mixture travel different distances based on how strongly they interact with the paper versus the solvent, allowing separation and identification. Paper chromatography is commonly used to analyze substances like amino acids, drugs, and biochemicals.