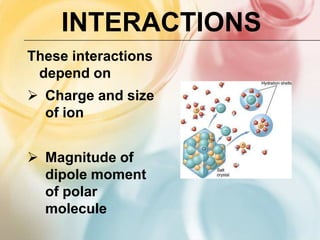
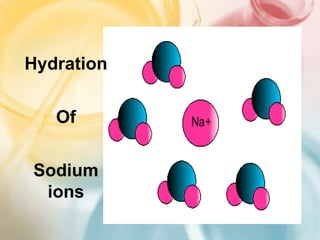
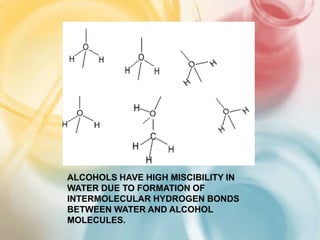
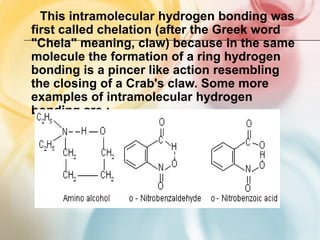
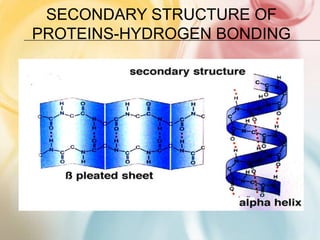
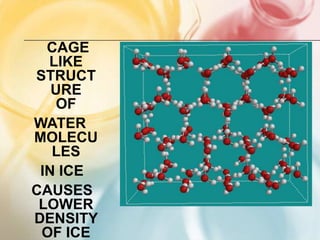
The document discusses different types of intermolecular forces including London dispersion forces, dipole-dipole forces, dipole-induced dipole forces, ion-dipole interactions, and hydrogen bonding. It provides examples of how these forces influence the properties of substances such as their melting and boiling points. Hydrogen bonding specifically is described as being an important intermolecular force that influences the properties of water, alcohols, and other polar molecules.