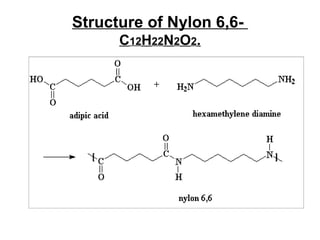
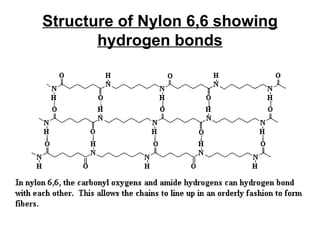
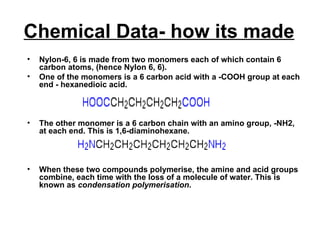
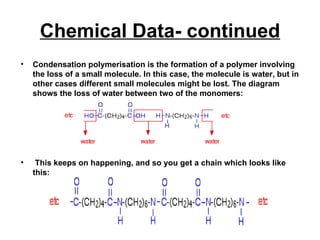
Nylon 6,6 is a type of polyamide polymer commonly known as nylon. It has a repeating molecular structure formed from the condensation polymerization of hexamethylenediamine and adipic acid, resulting in the loss of water. Nylon 6,6 has various desirable physical properties such as stability, strength, and resistance to water, heat, and chemicals, making it suitable for applications such as tires, ropes, parachutes, swimwear, and machine parts.