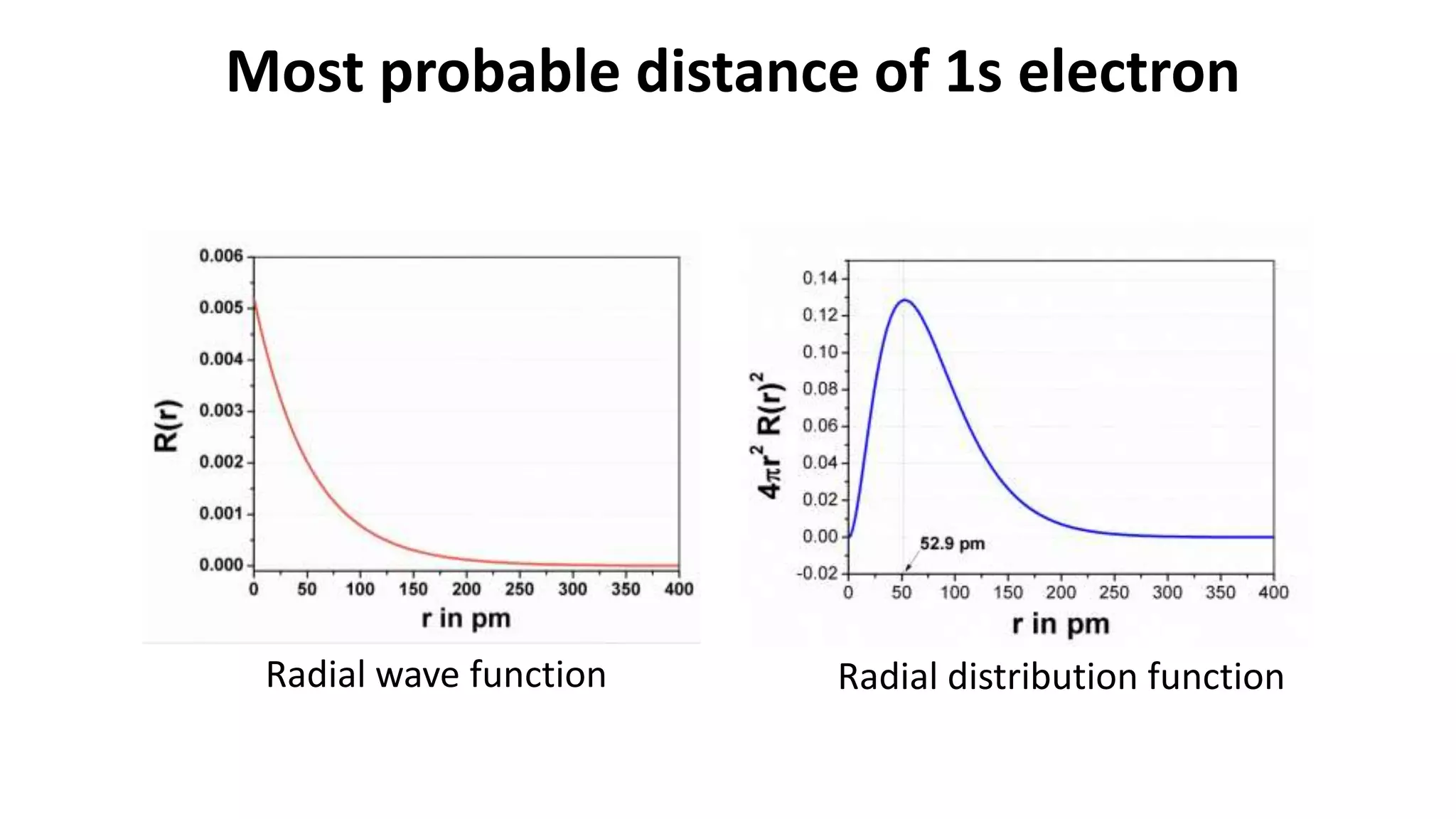
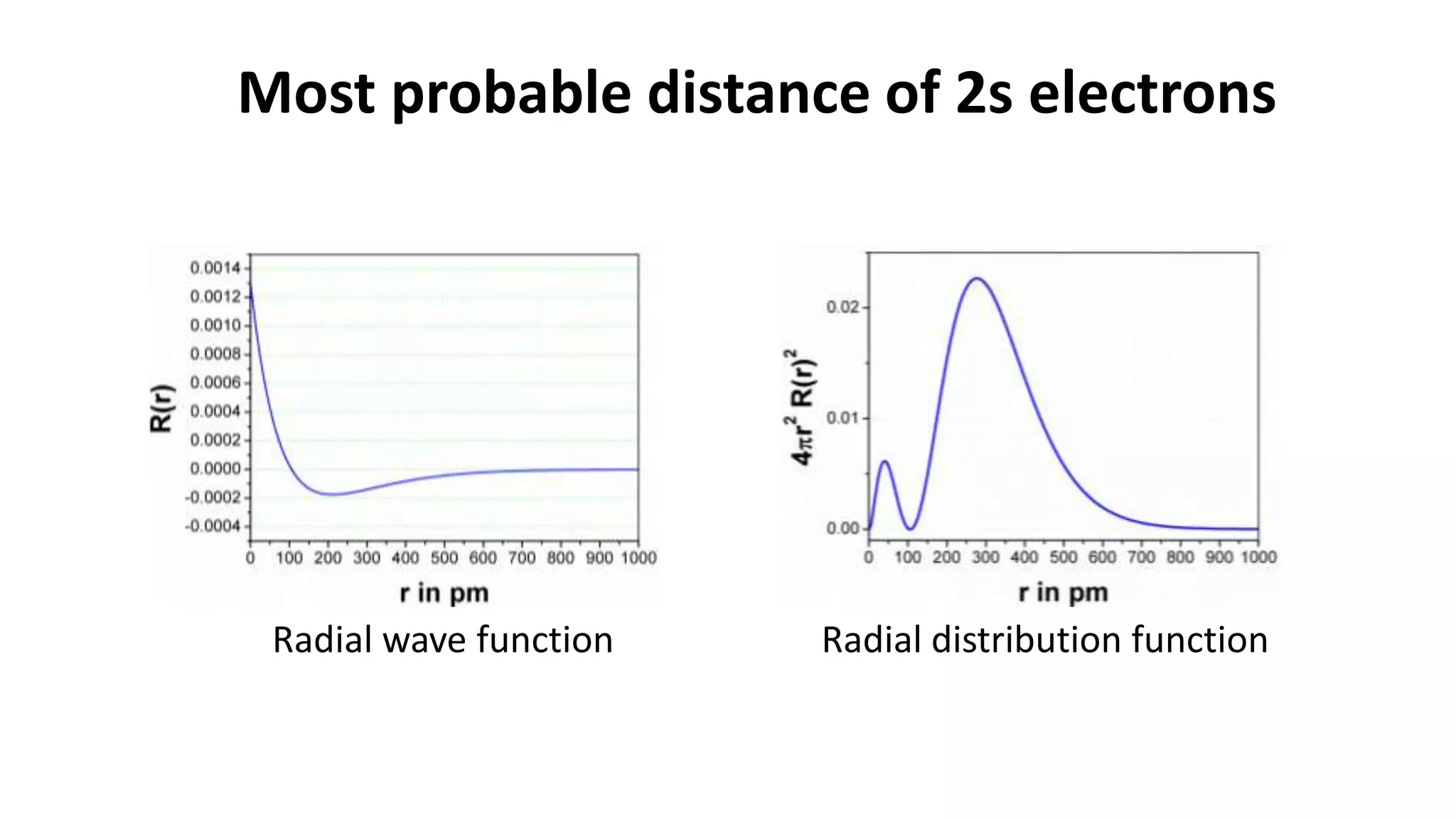
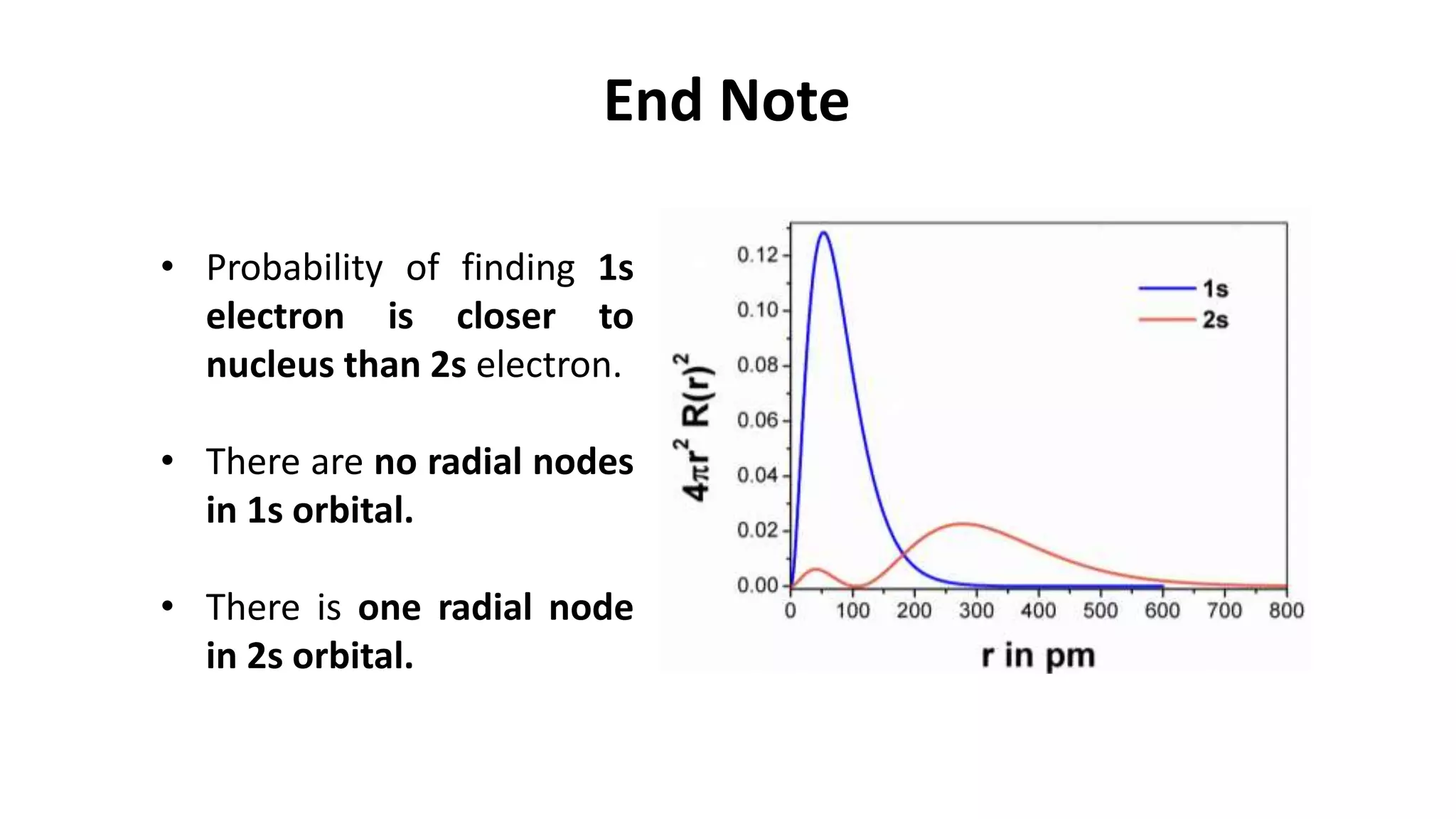
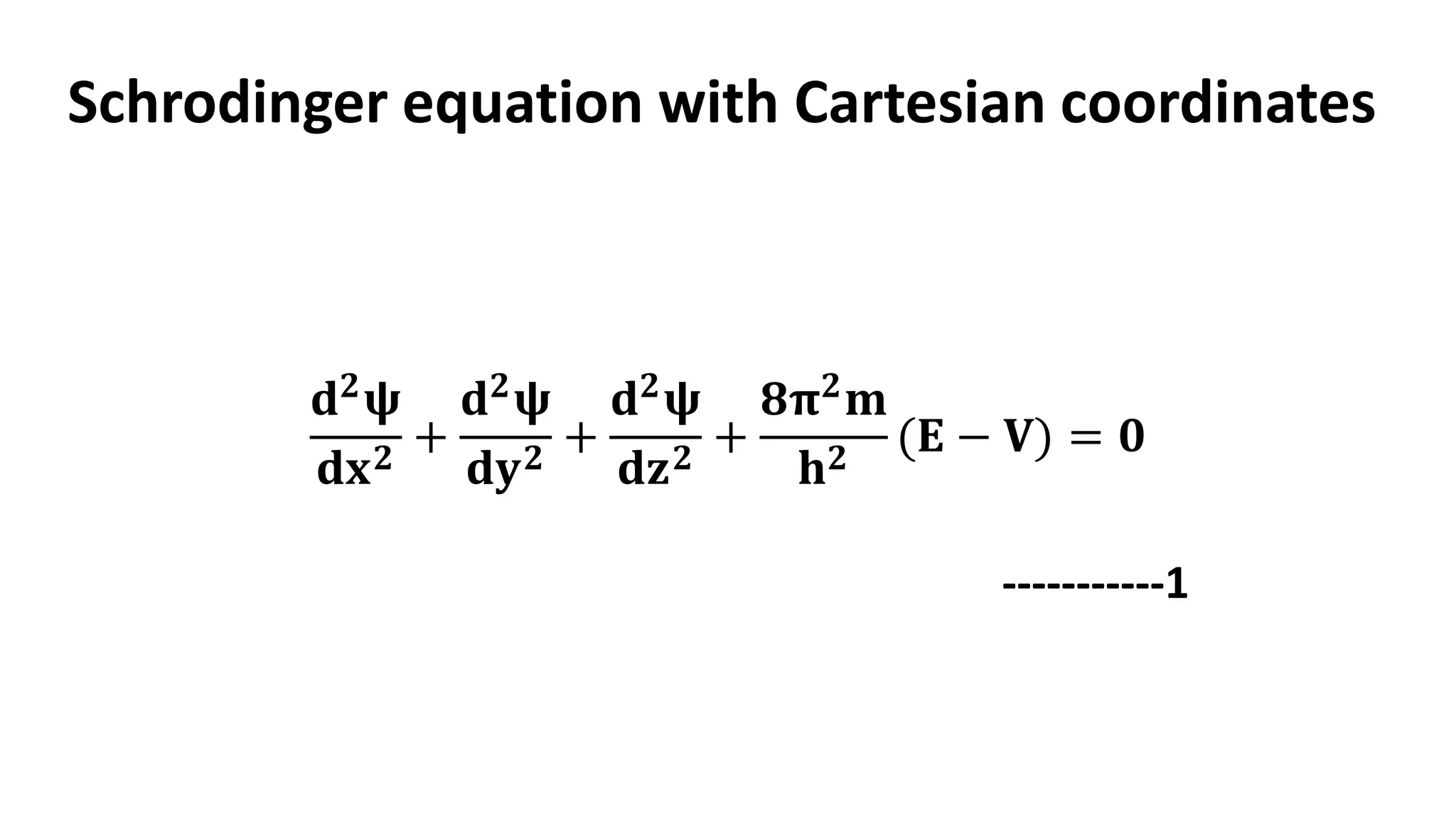
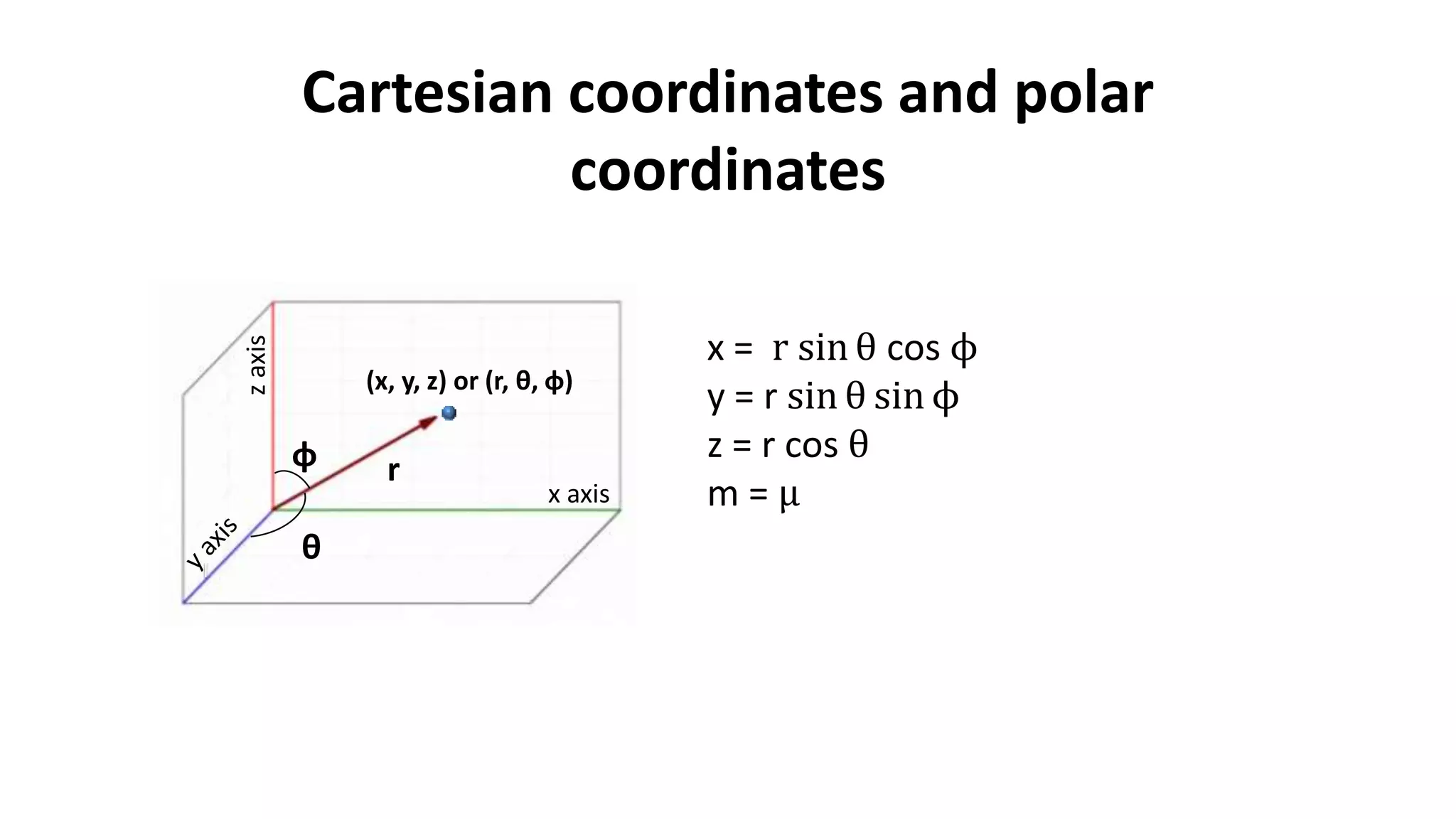
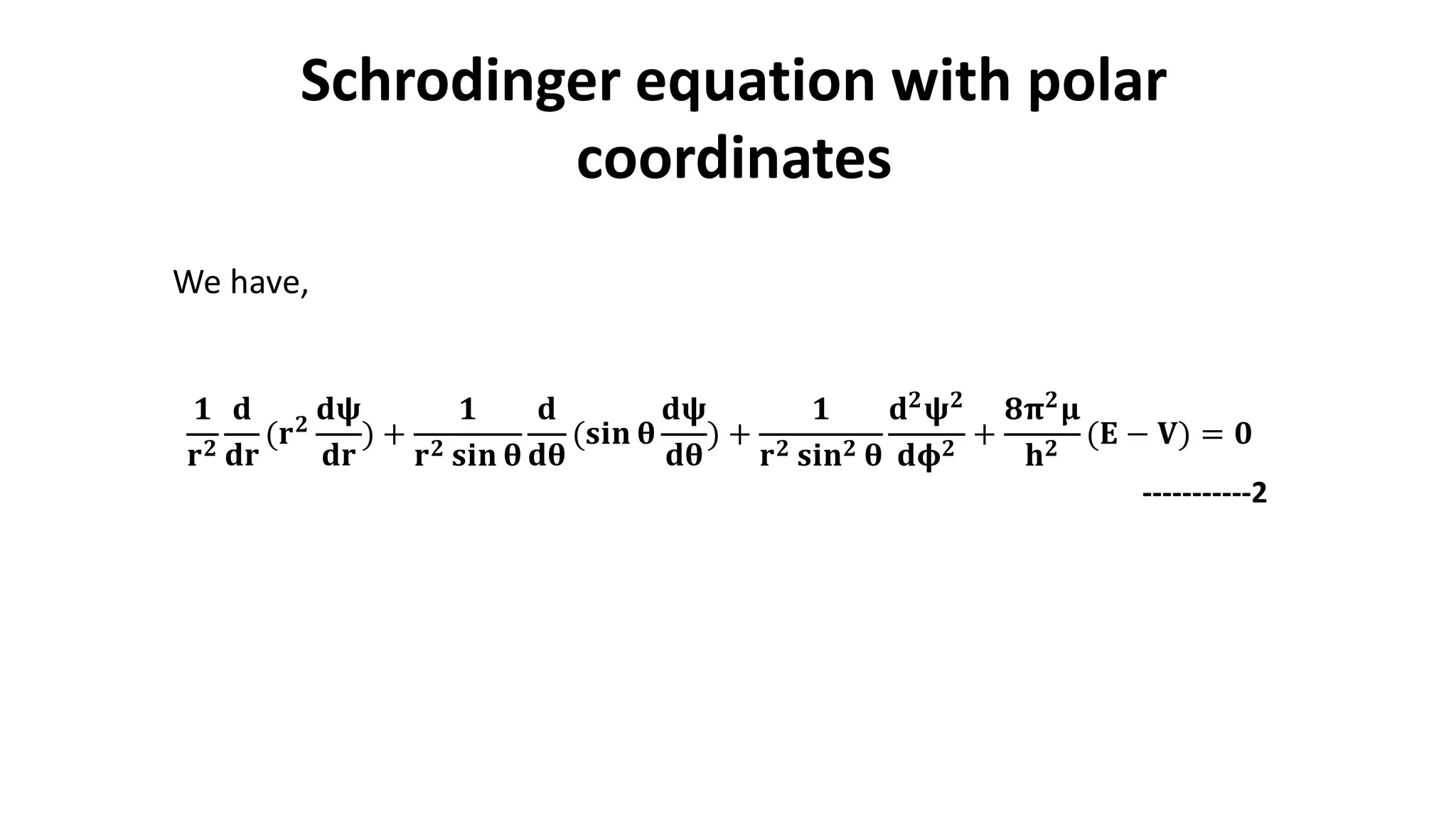
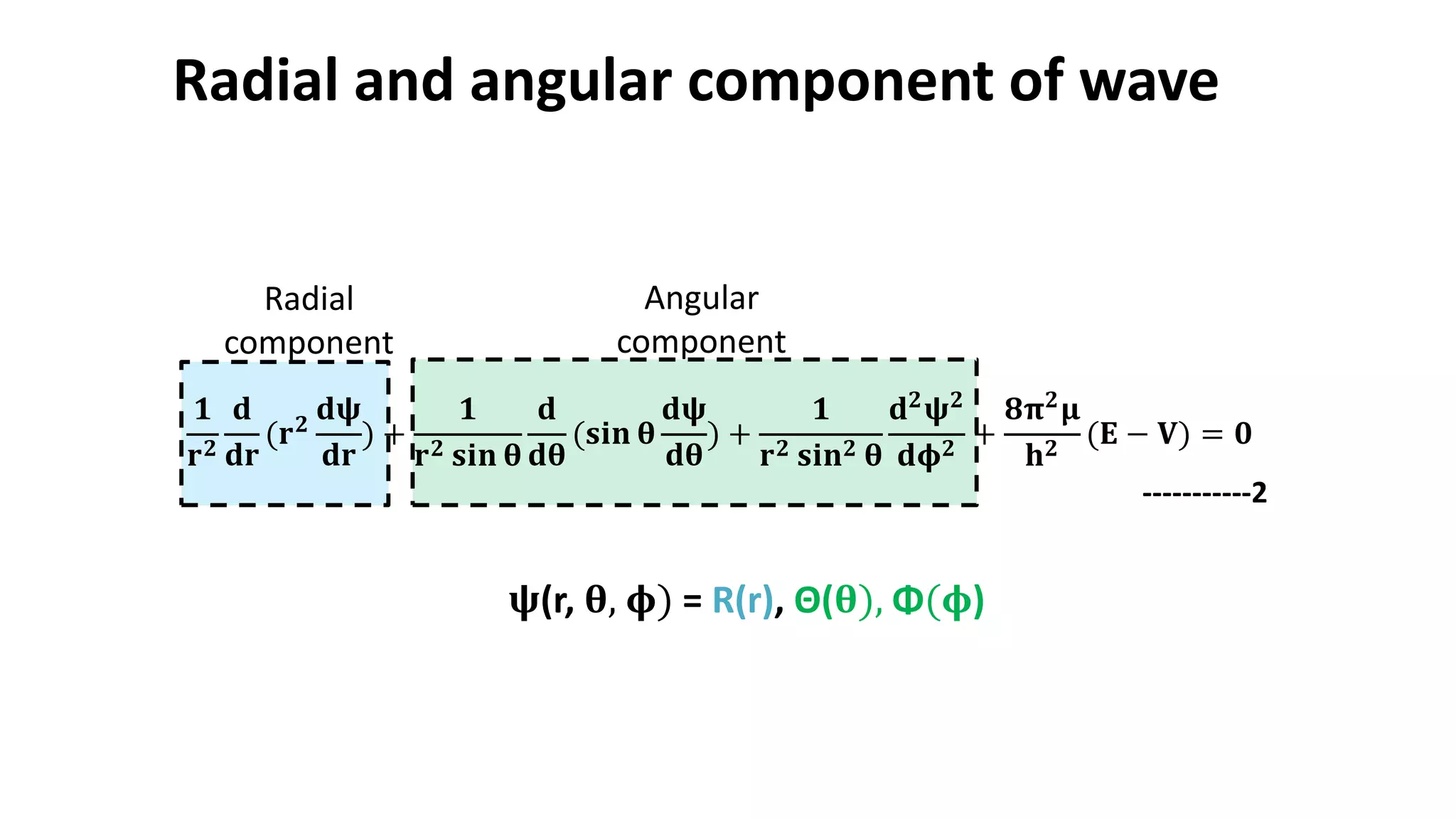
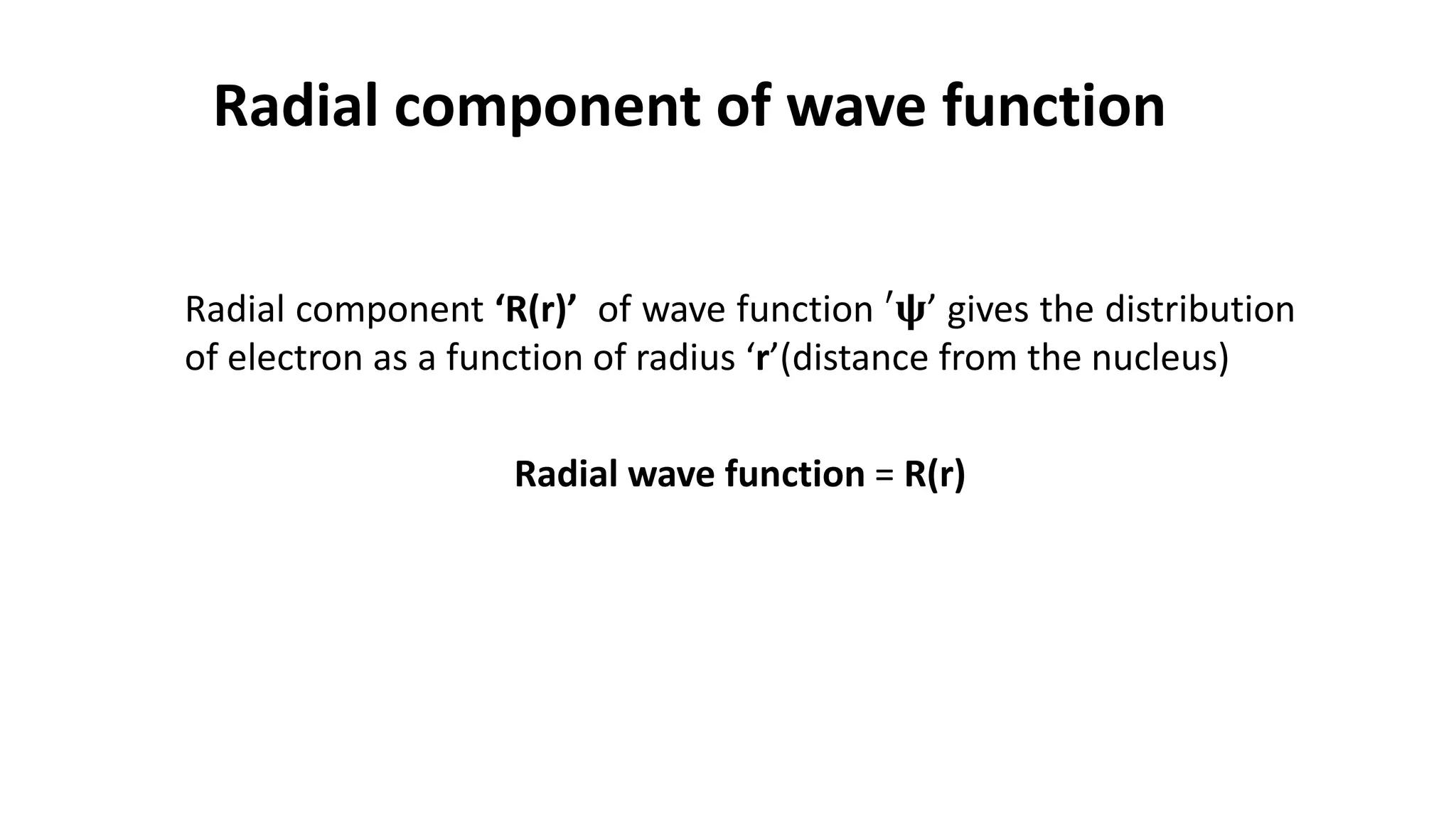
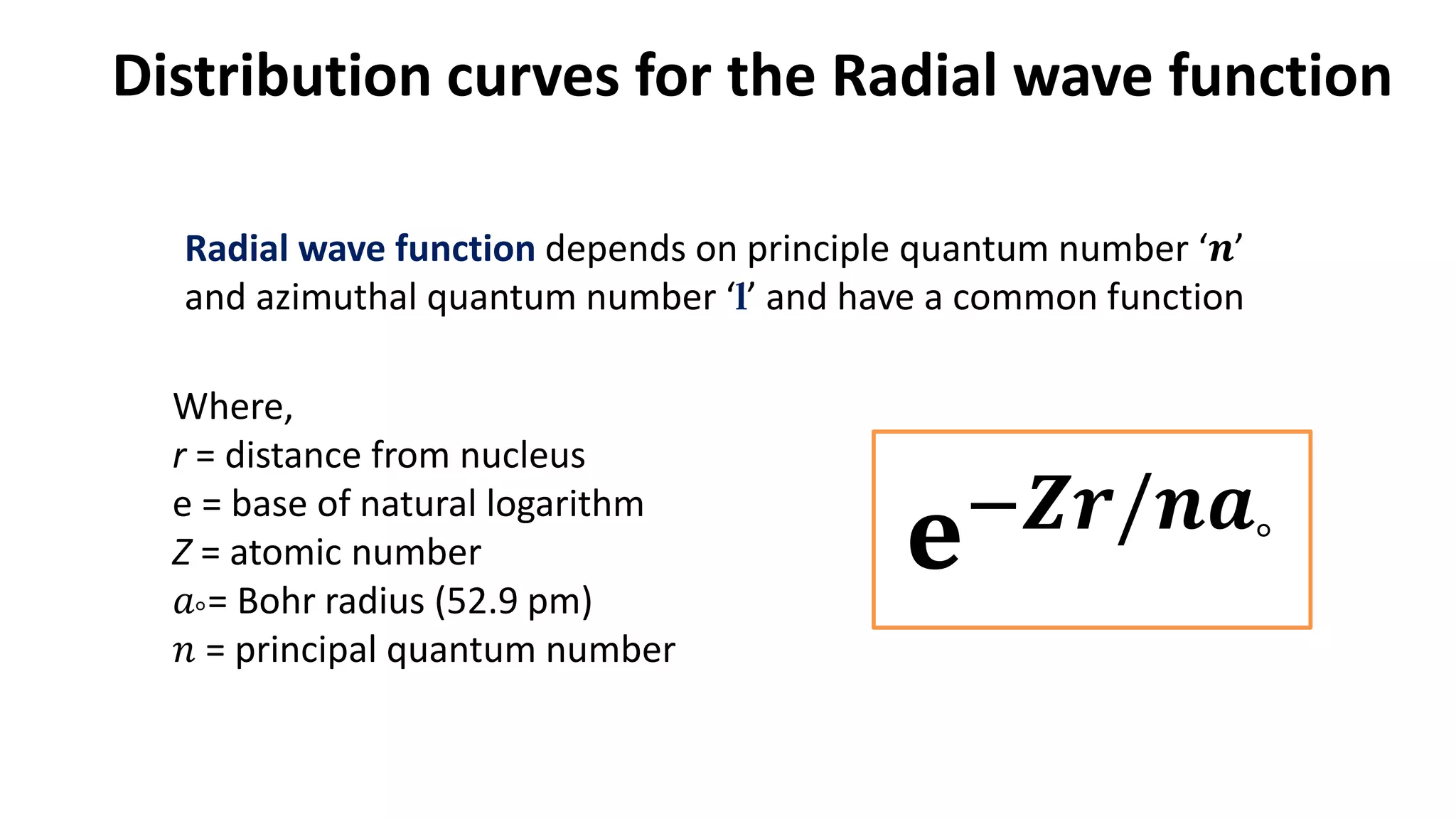
The document discusses the radial and angular parts of the hydrogenic wave functions. It shows how the Schrodinger equation can be written using both Cartesian and polar coordinates. The radial component R(r) of the wave function gives the distribution of electrons as a function of radius r from the nucleus. R(r) depends on the principal quantum number n and azimuthal quantum number l. Graphs and equations are provided to show the radial wave functions and most probable distances for 1s and 2s orbitals.

![Orbital
Quantum
number
Radial component of wave function
‘R(r)’
1s n = 1, l = 0 2(
𝑧
𝑎°
)3/2
𝑒−𝑍𝑟/𝑛𝑎°
2s n = 2, l = 0
1
8
1
2
𝑧
𝑎°
3
2
2 −
2𝑍r
n𝑎°
𝑒−𝑍𝑟/𝑛𝑎°
‘[R(r)]2’ gives the probability of finding electron.
Distribution curves for the Radial wave function](https://image.slidesharecdn.com/radialdistributionfunctionsrevised-211016054608/75/Radial-distribution-function-and-most-probable-distance-of-1s-and-2s-electron-8-2048.jpg)
![r in pm R(r) [R(r)]2 4πr2 [R(r)]2
0 0.005198122 2.70205E-05 0
52.5 0.001926797 3.71255E-06 0.128522758
52.9 0.001912282 3.65682E-06 0.128530144
53.5 0.001890715 3.5748E-06 0.128513735
224.8 7.41823E-05 5.50301E-09 0.003492872
R(r) =2(
𝑧
𝑎°
)3/2
𝑒−𝑍𝑟/𝑛𝑎°
Most probable distance of 1s electron](https://image.slidesharecdn.com/radialdistributionfunctionsrevised-211016054608/75/Radial-distribution-function-and-most-probable-distance-of-1s-and-2s-electron-9-2048.jpg)