Embed presentation
Downloaded 109 times

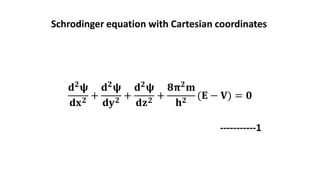


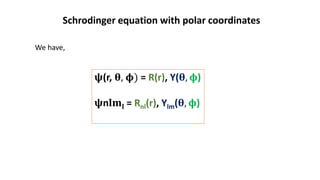
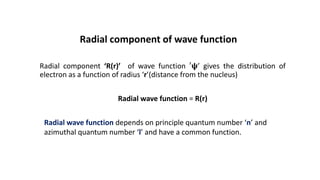
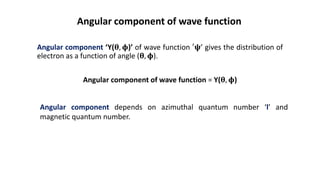


This document discusses the radial and angular parts of the hydrogenic wave functions for the 1s, 2s, 2p, 3s, 3p and 3d orbitals. It explains that the radial component, R(r), gives the distribution of the electron as a function of radius r from the nucleus. The angular component, Y(θ,φ), gives the distribution as a function of the angles θ and φ. It describes how R(r) depends on the principal and azimuthal quantum numbers, while Y(θ,φ) depends on the azimuthal and magnetic quantum numbers. Graphs are presented showing the radial variation of the wave functions for different orbitals.









