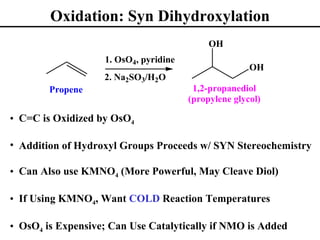
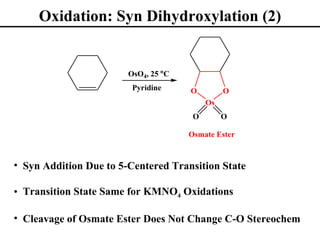
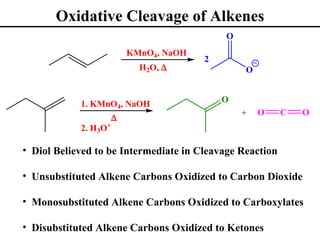
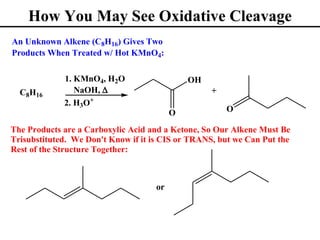
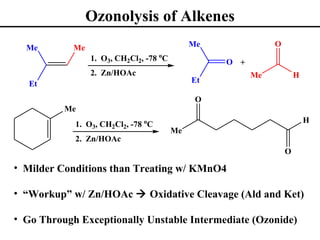
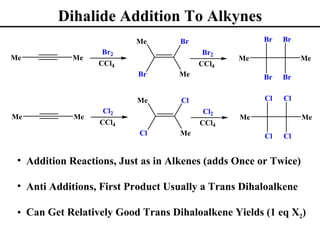
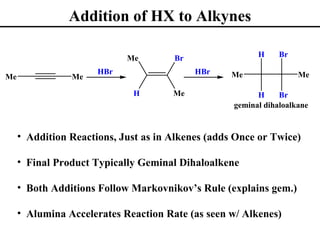
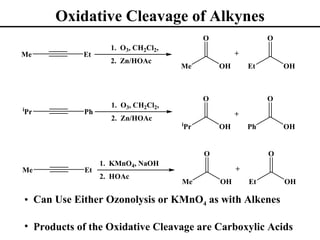
The document outlines various addition reactions to alkenes and alkynes, including addition of HX, H2O, SO4, halogens, and oxidation reactions. It discusses important concepts like Markovnikov's rule, regioselectivity, stereochemistry including anti-Markovnikov additions from hydroboration-oxidation. A variety of methods are covered for converting alkenes and alkynes to alcohols, diols, dihalides, and cleaving carbon-carbon double or triple bonds.
![Addition Reactions
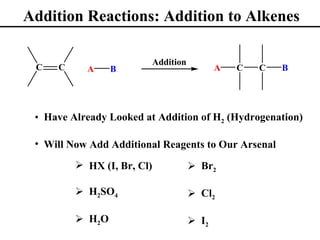
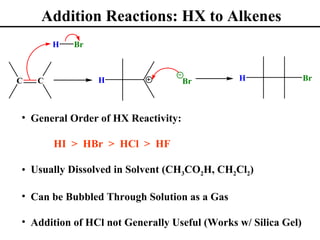
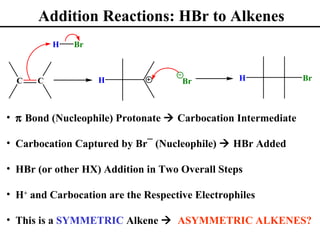
• Addition Reactions to Alkenes (Section 8.1)
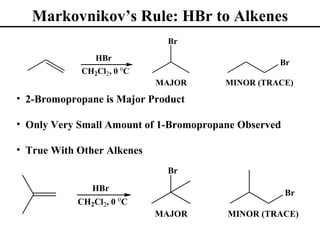
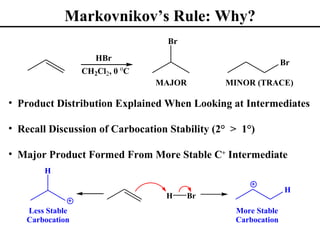
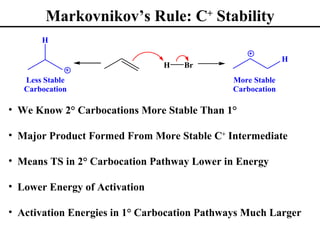
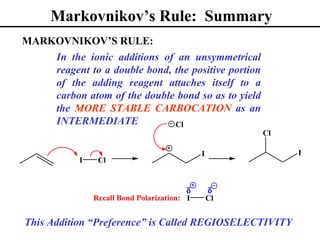
• Markovnikov’s Rule (Section 8.2)
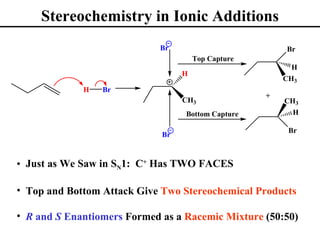
• Stereochemistry of Ionic Addition to Alkenes (Section 8.3)
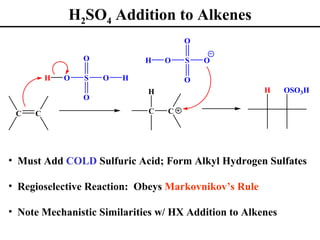
• H2SO4 Additions to Alkenes (Section 8.4)
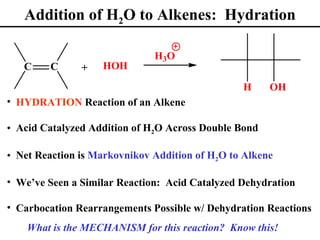
• H2O Additions to Alkenes (Section 8.5)
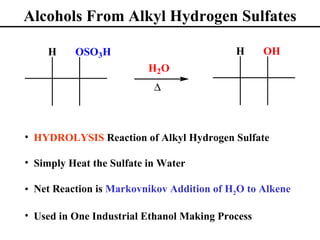
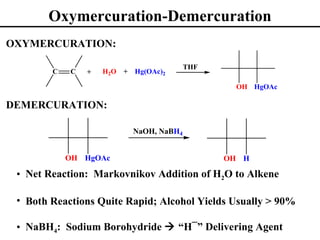
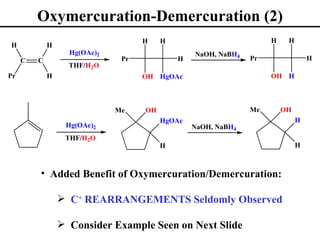
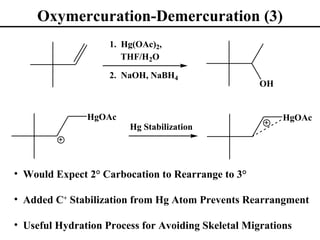
• Oxymercuration/Demurcuration (Section 8.6)
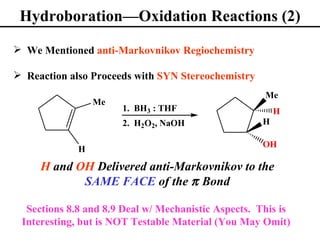
• Hydroboration/Oxidation (Section 8.7)
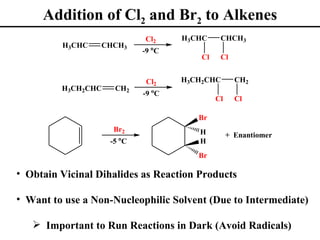
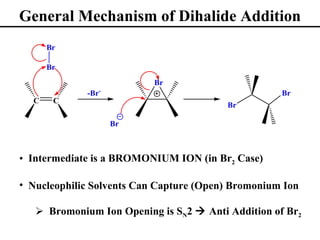
• Addition of Br2 and Cl2 to Alkenes (Section 8.12)
• Stereochemistry of Dihalide Additions (Section 8.13)
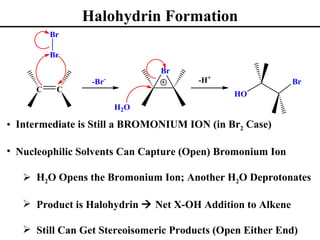
• Halohydrin Formation [Net Addition of X-OH] (Section 8.14)
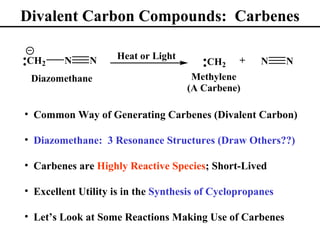
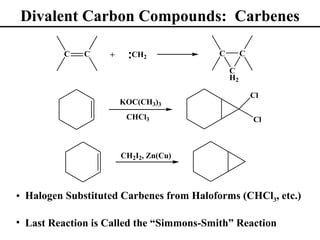
• Divalent Carbon Compounds: Carbenes (Section 8.15)
• Oxidations of Alkenes (Sections 8.16-8.17)
• Additions to Alkynes (Sections 8.18-8.19)
• Oxidative Cleavage of Alkynes (Section 8.20)
• Applications in Synthesis (Section 8.21)](https://image.slidesharecdn.com/chemistry343chapter8-120418043540-phpapp01/75/Chemistry-343-chapter_8-1-2048.jpg)