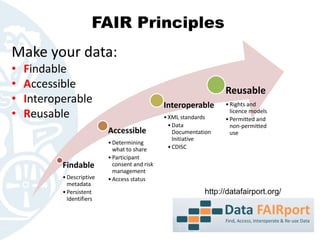
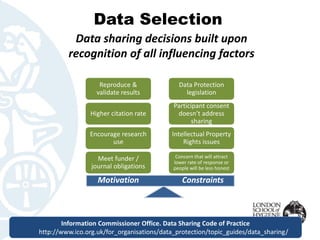
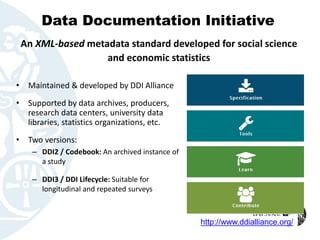
The document outlines the FAIR principles for data sharing, emphasizing that data should be Findable, Accessible, Interoperable, and Reusable. It discusses the importance of descriptive metadata, participant consent, and intellectual property rights while identifying key motivators such as research validation and funder requirements for data sharing. Furthermore, the document highlights the need for proper data documentation and standards, as well as the benefits of making data available for enhancing citation rates in research.