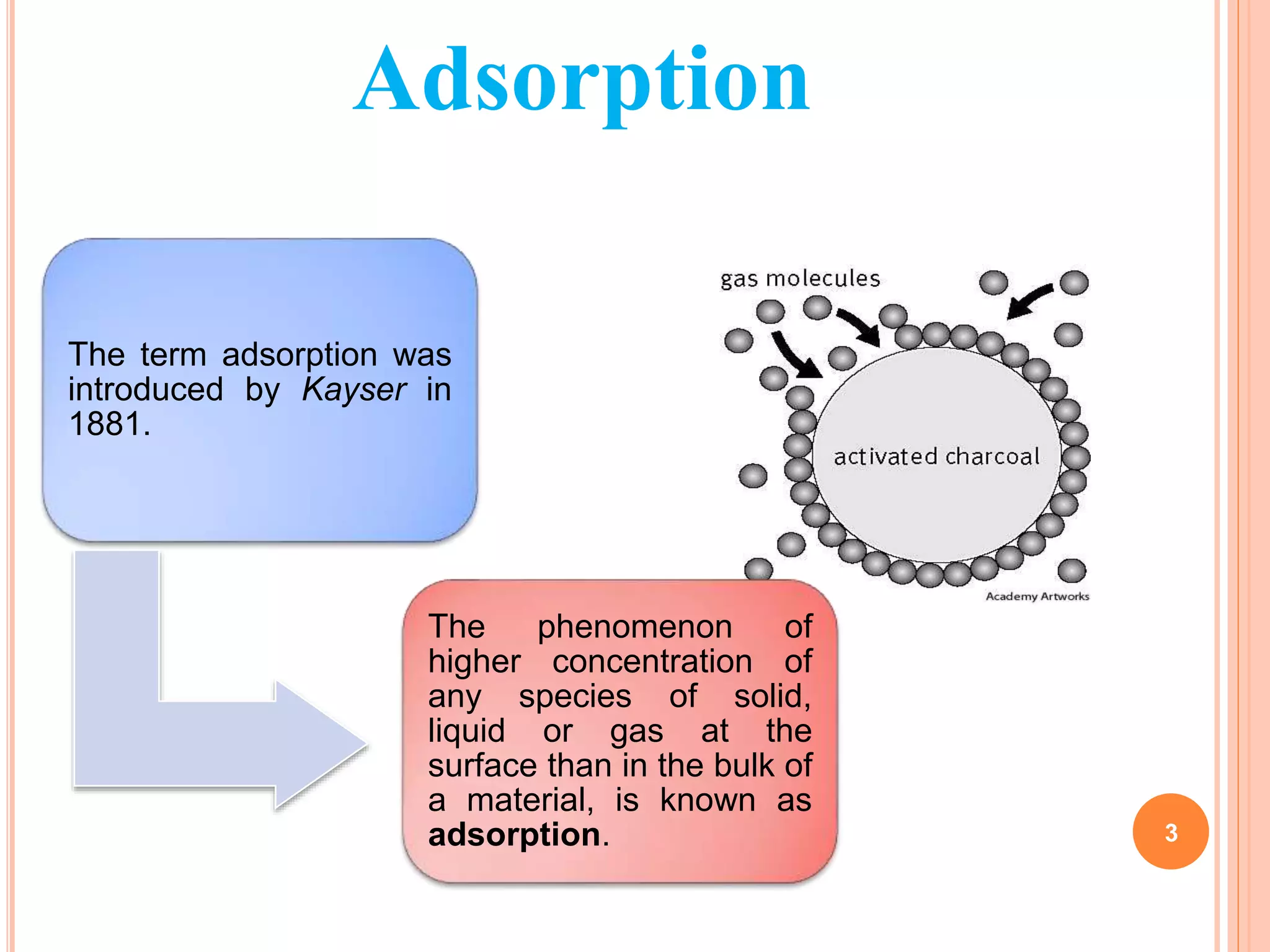
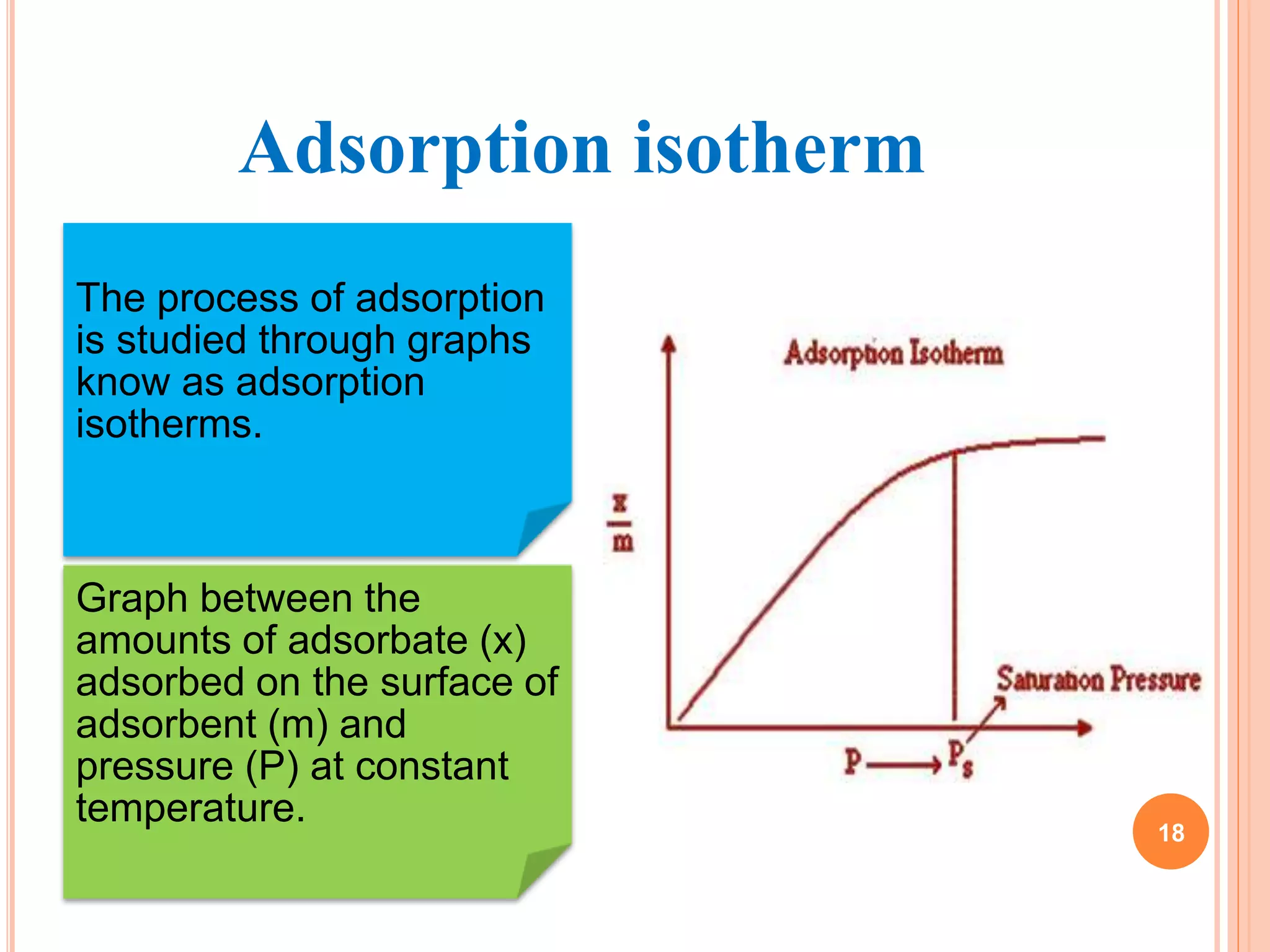
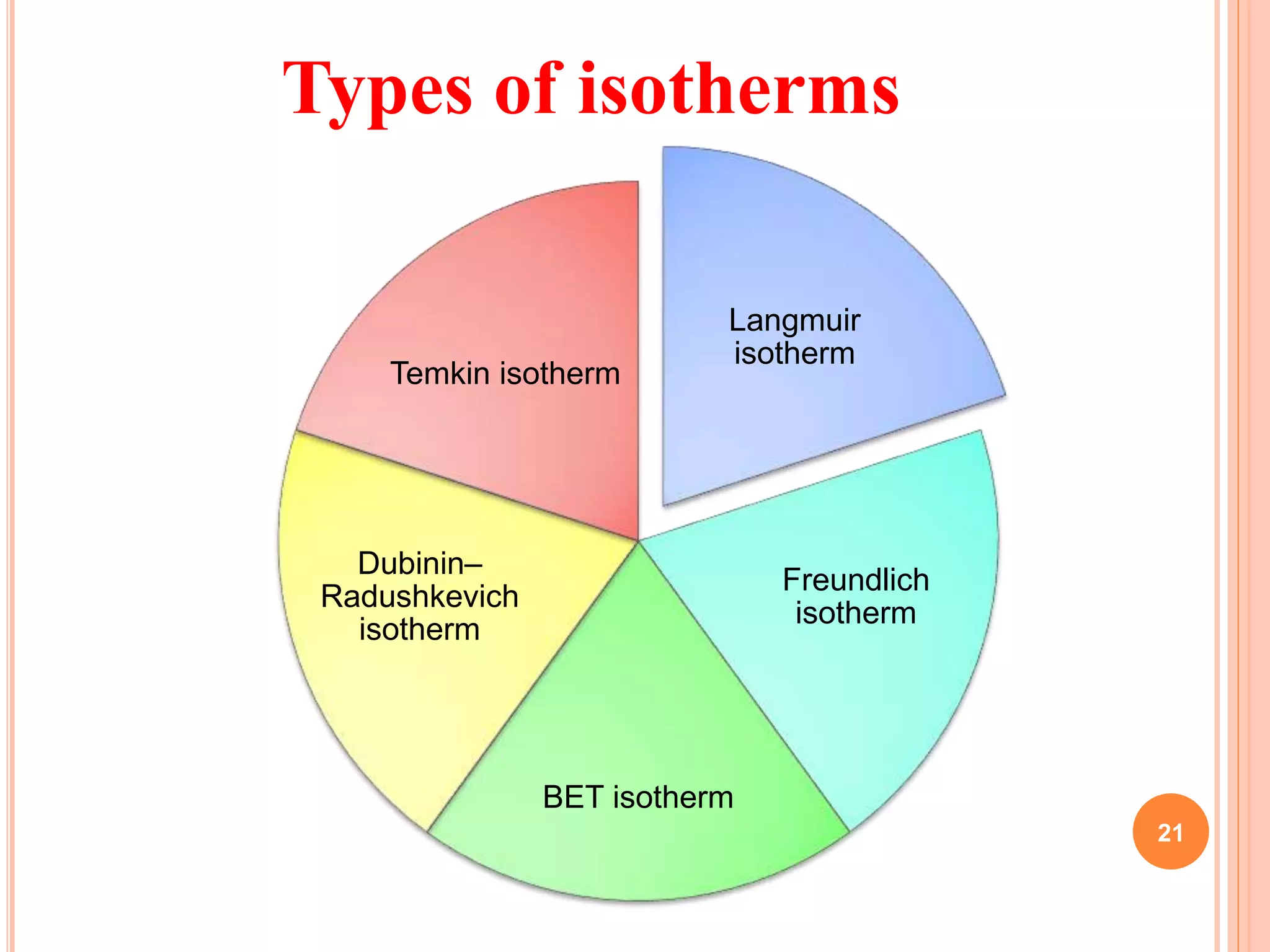
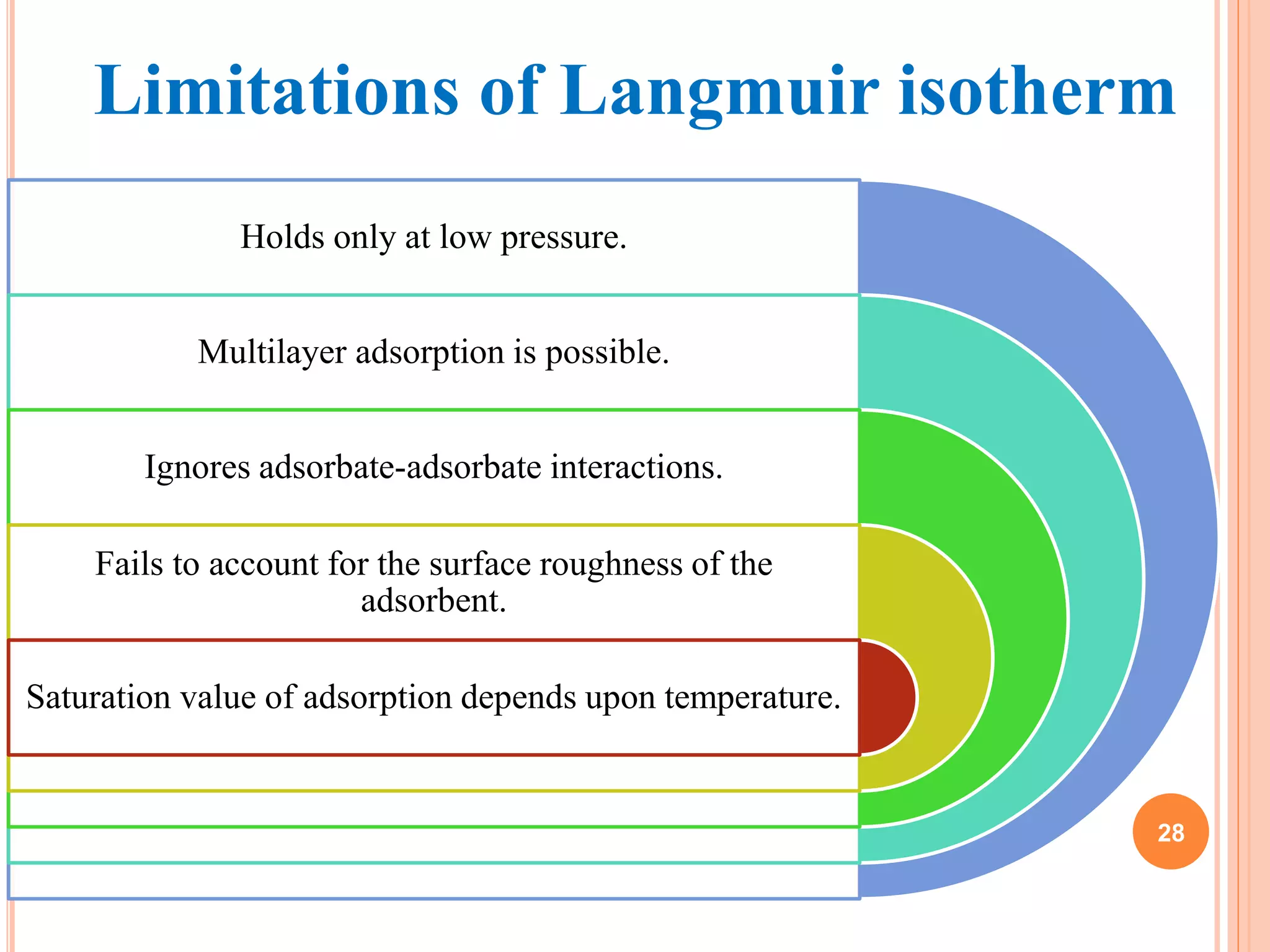
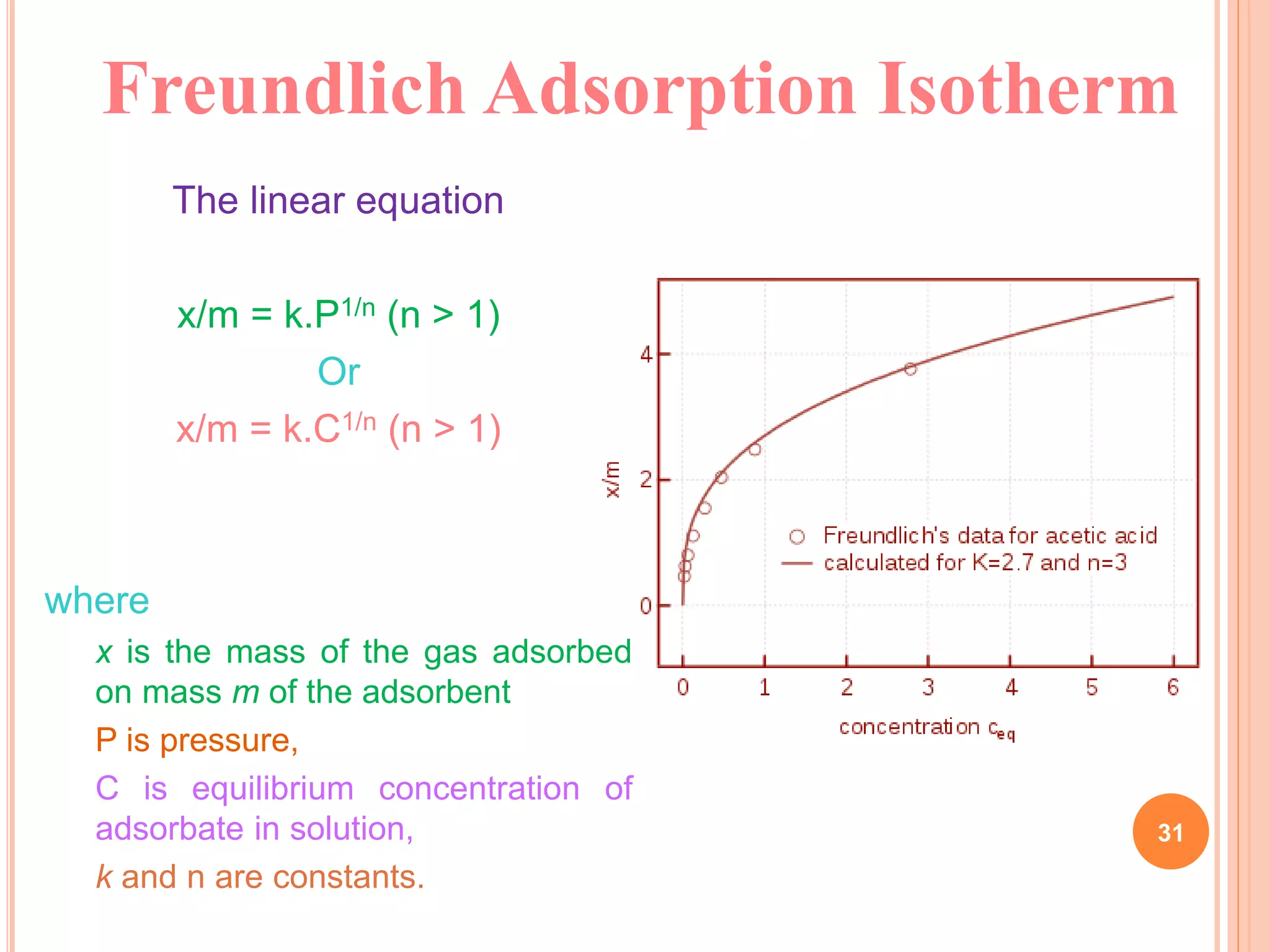
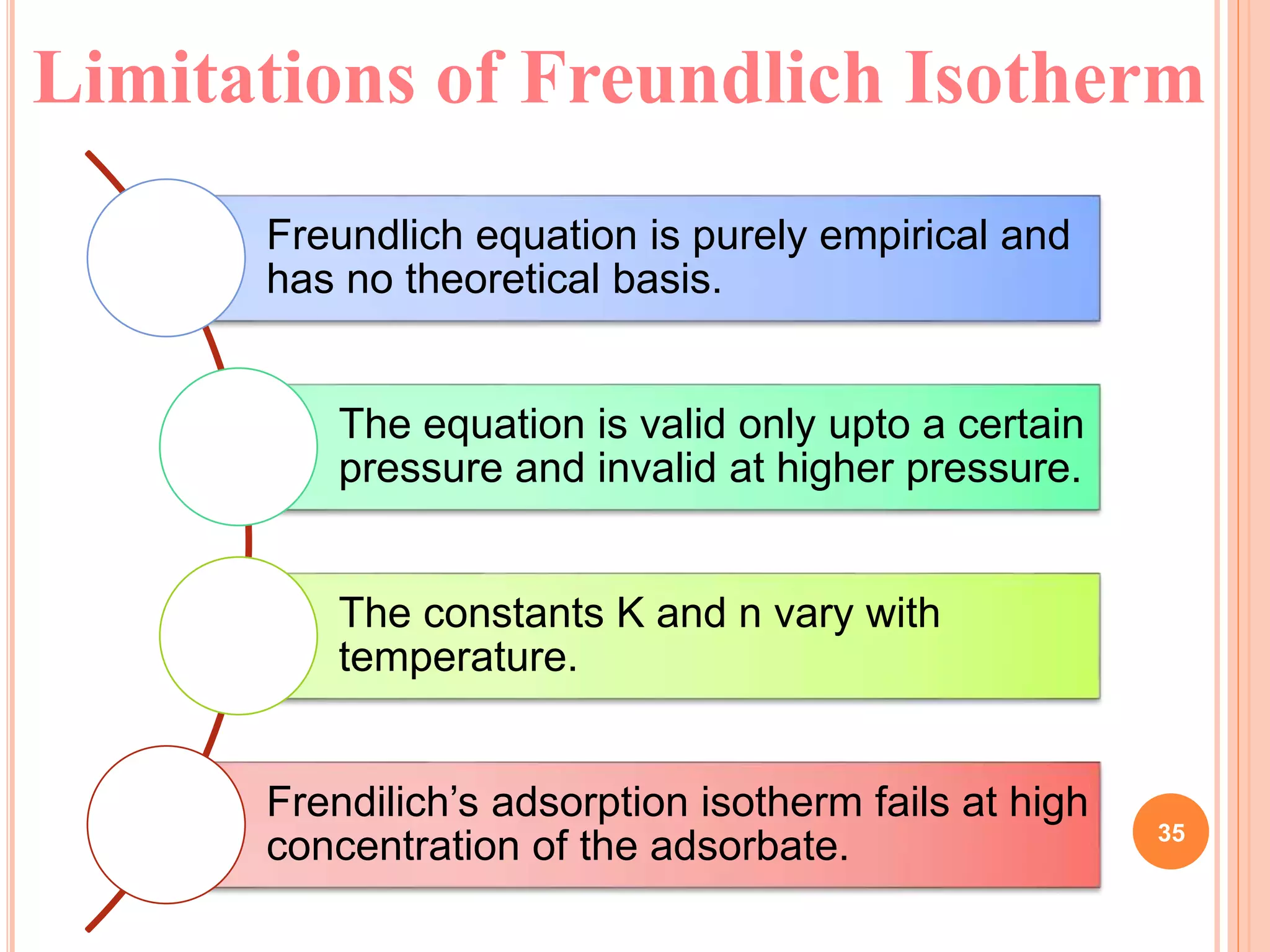
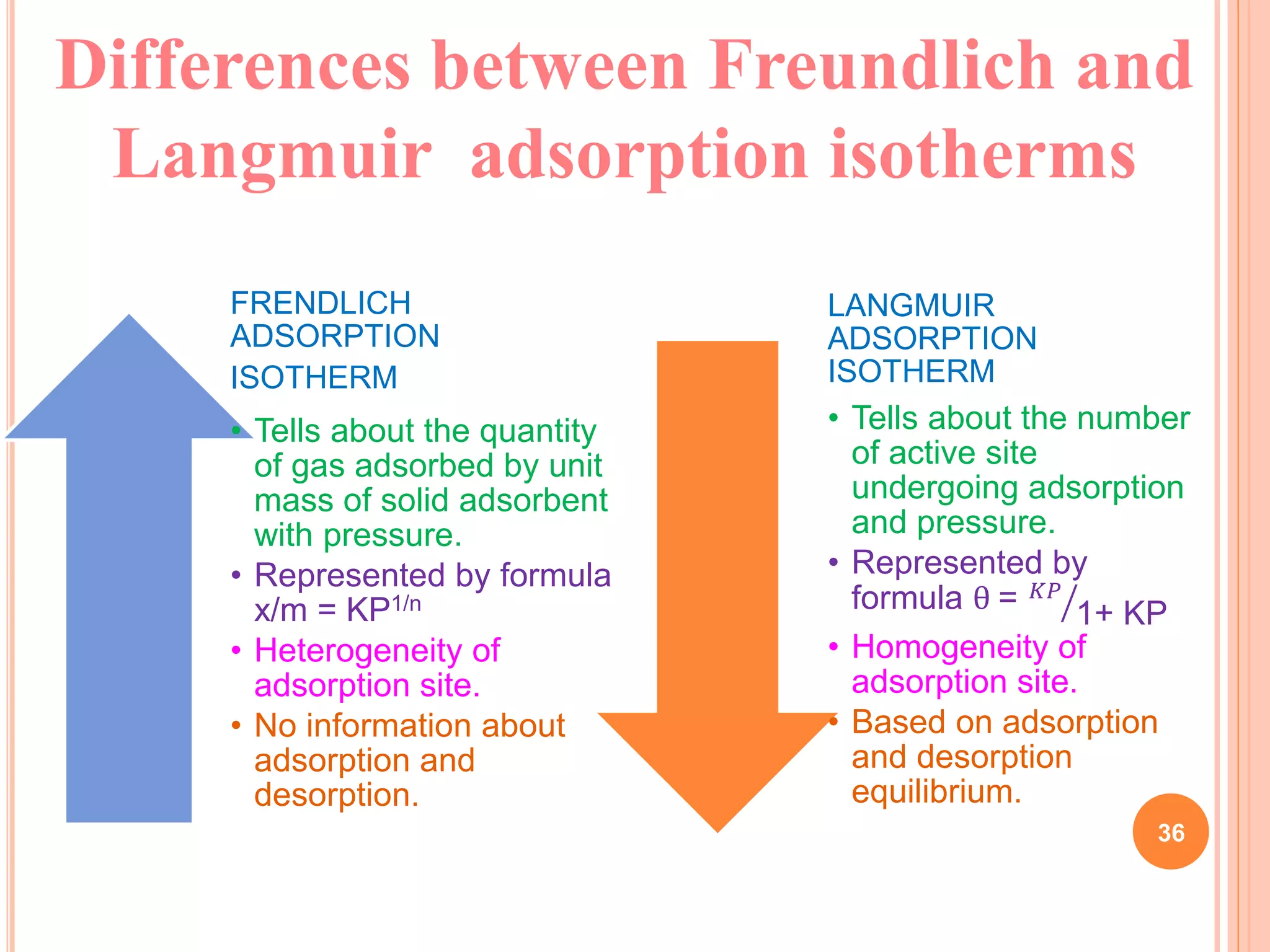
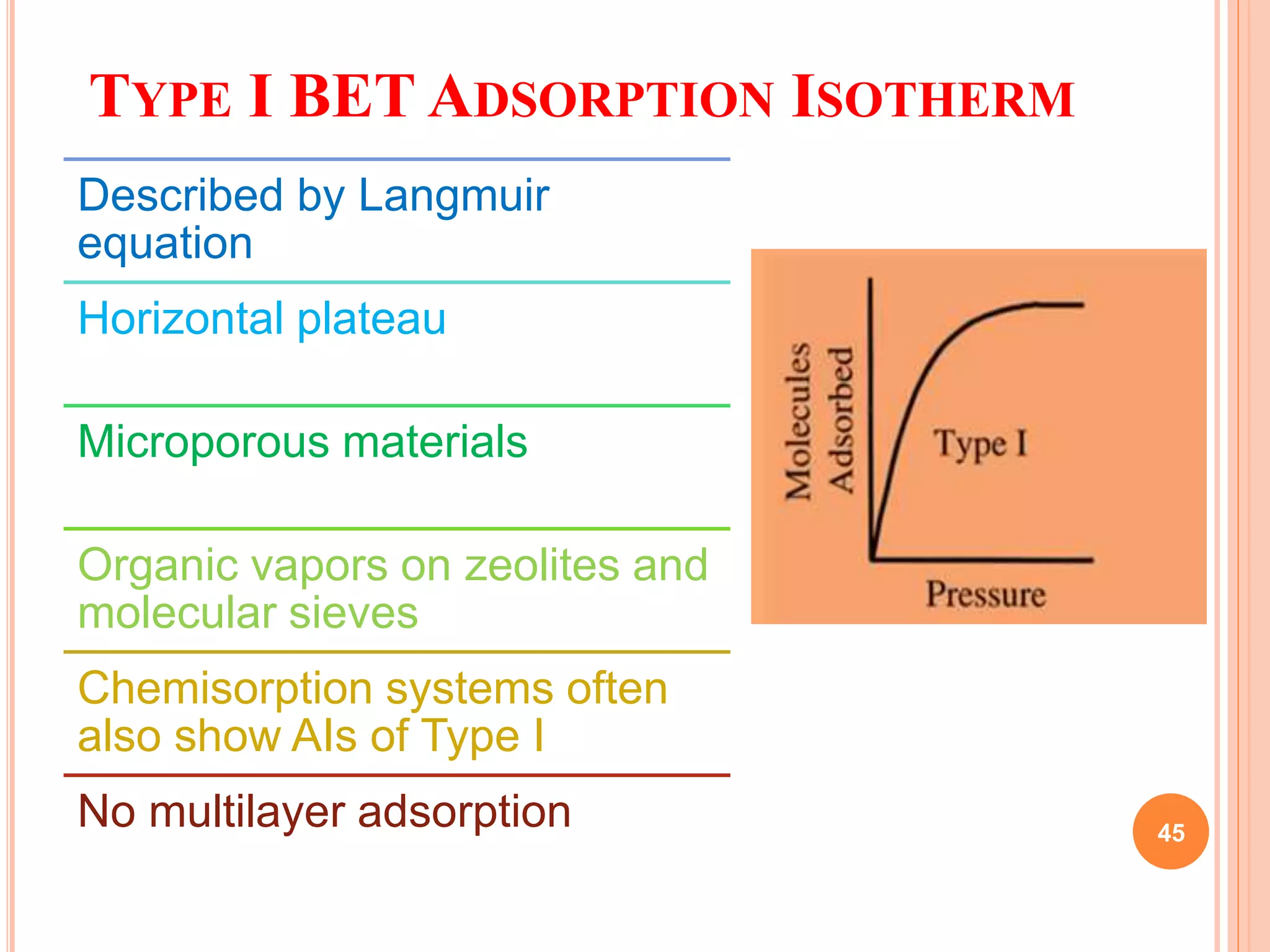
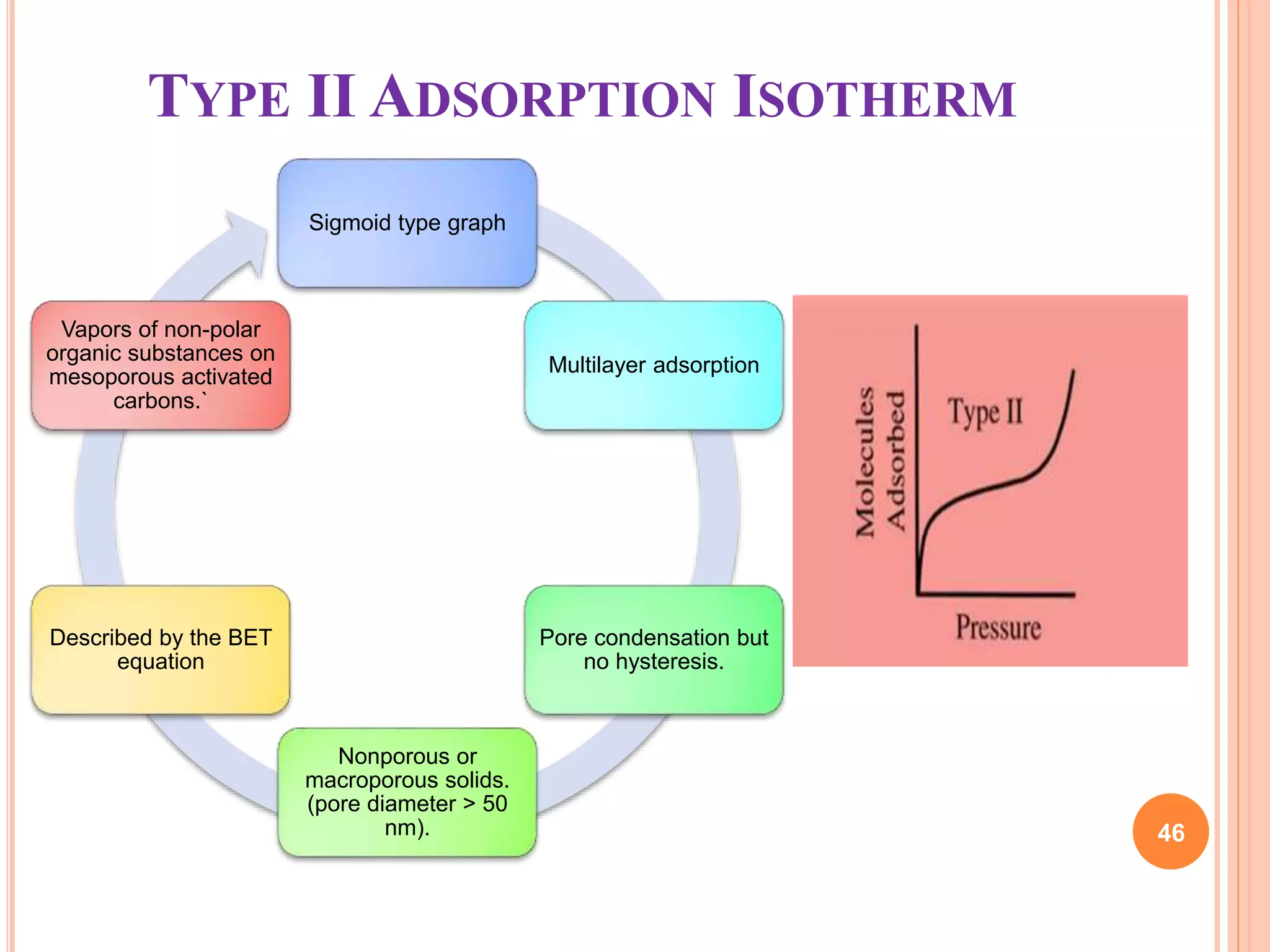
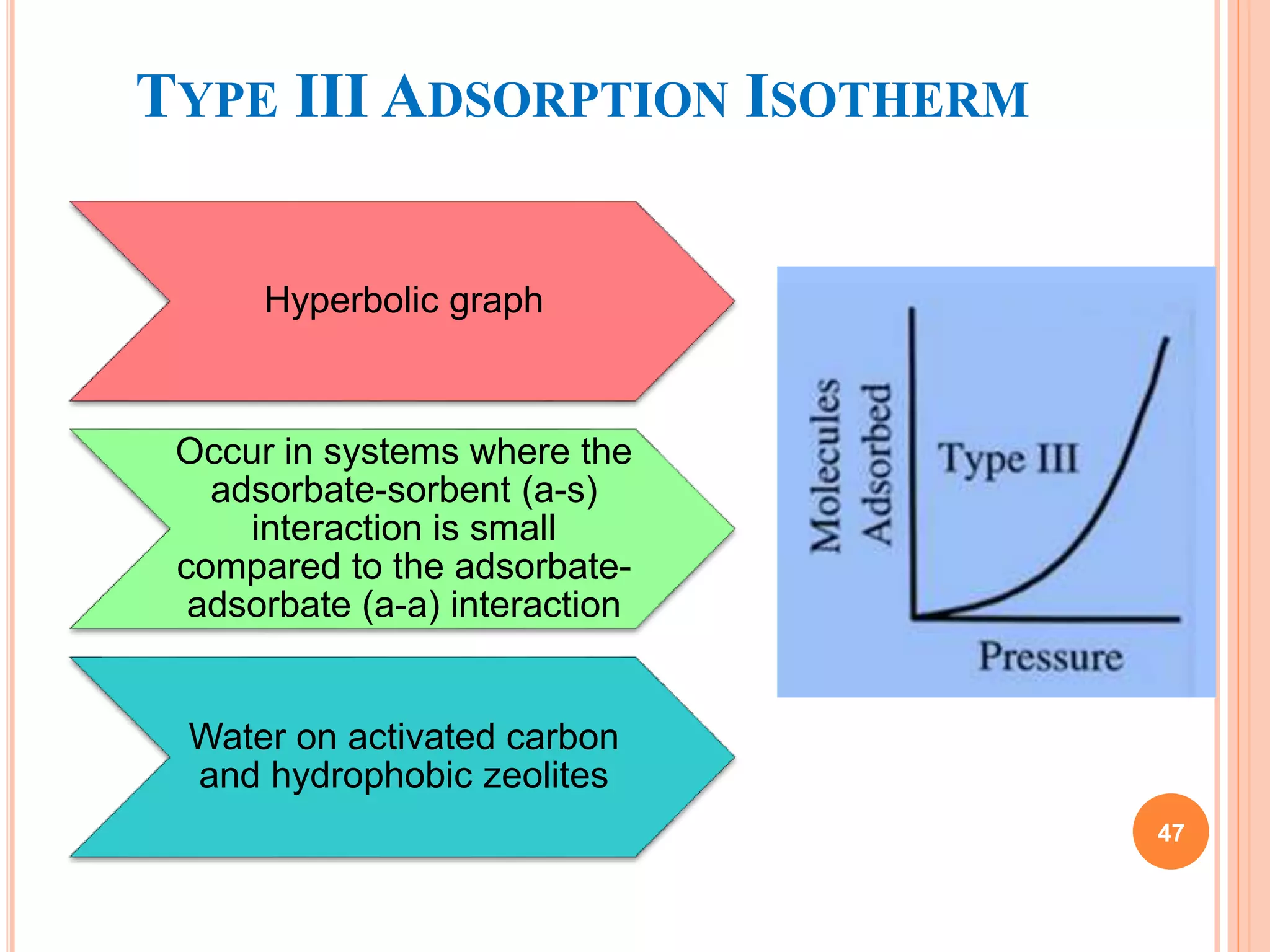
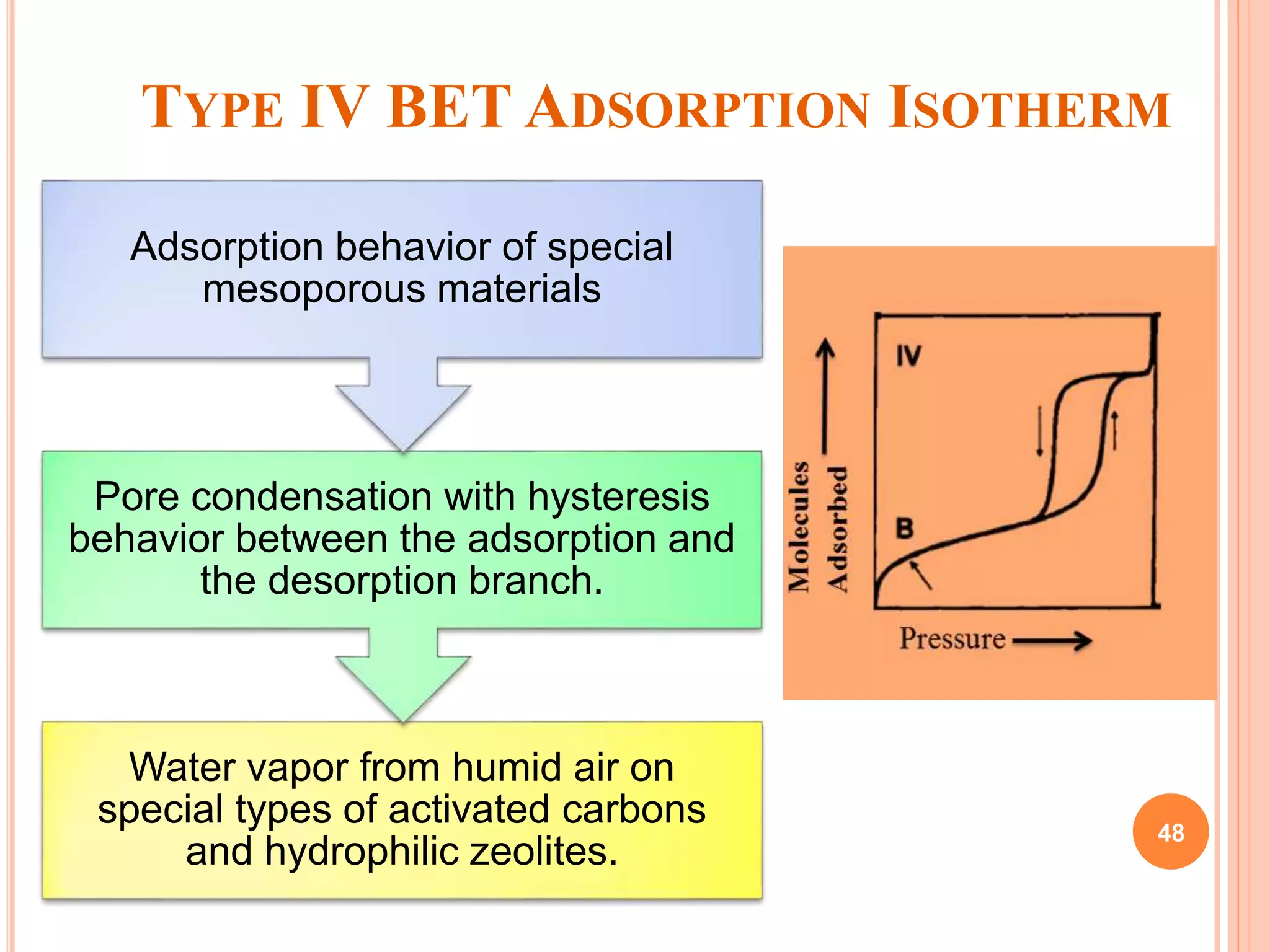
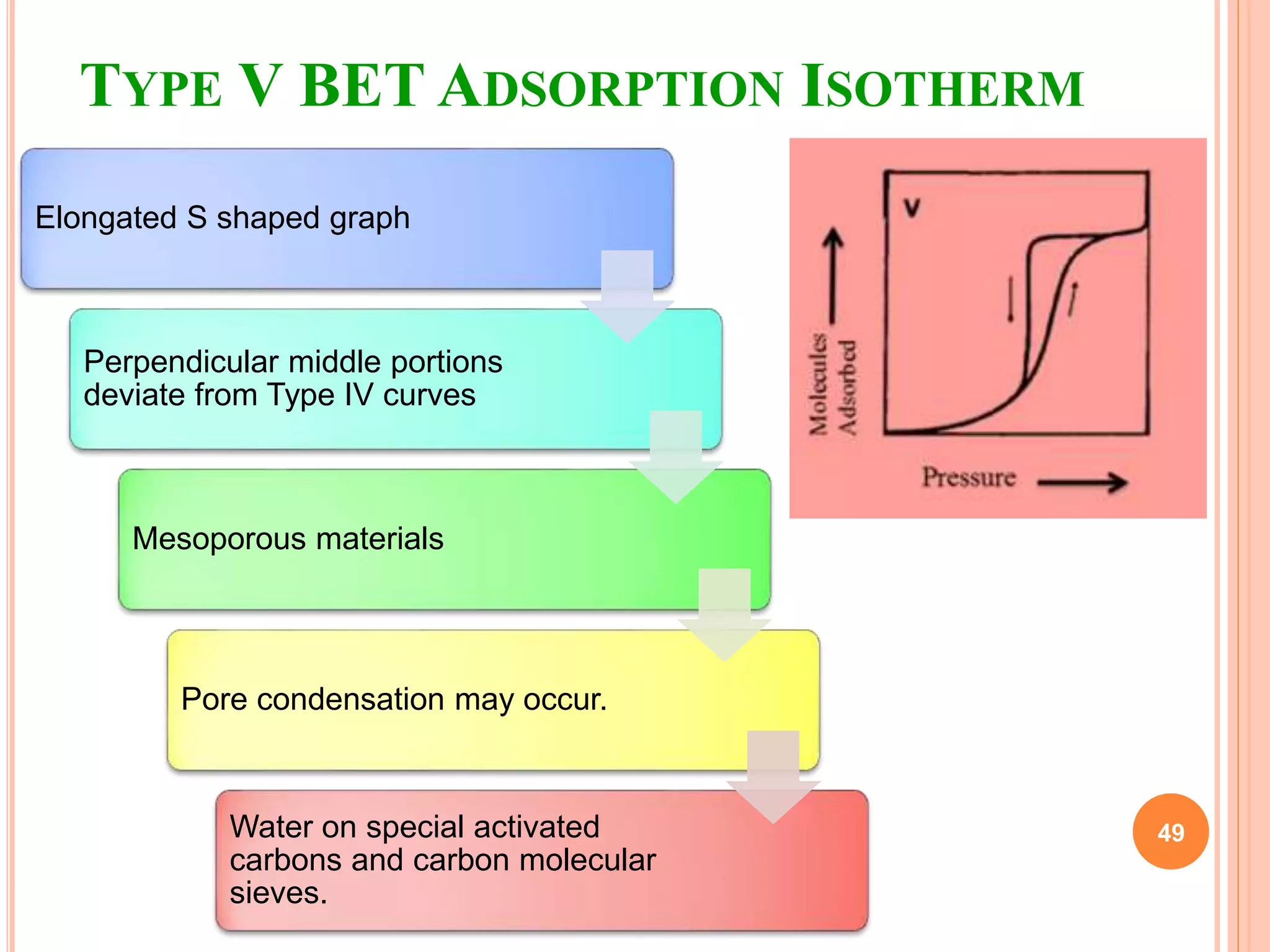
The document discusses the concept of adsorption, its mechanisms, types, and applications. It covers various adsorption isotherms, including Langmuir, Freundlich, BET, Dubinin-Radushkevich, and Temkin, explaining their equations, assumptions, and limitations. Additionally, it highlights factors that influence adsorption and its practical applications in areas such as gas masks, purification, and chromatography.