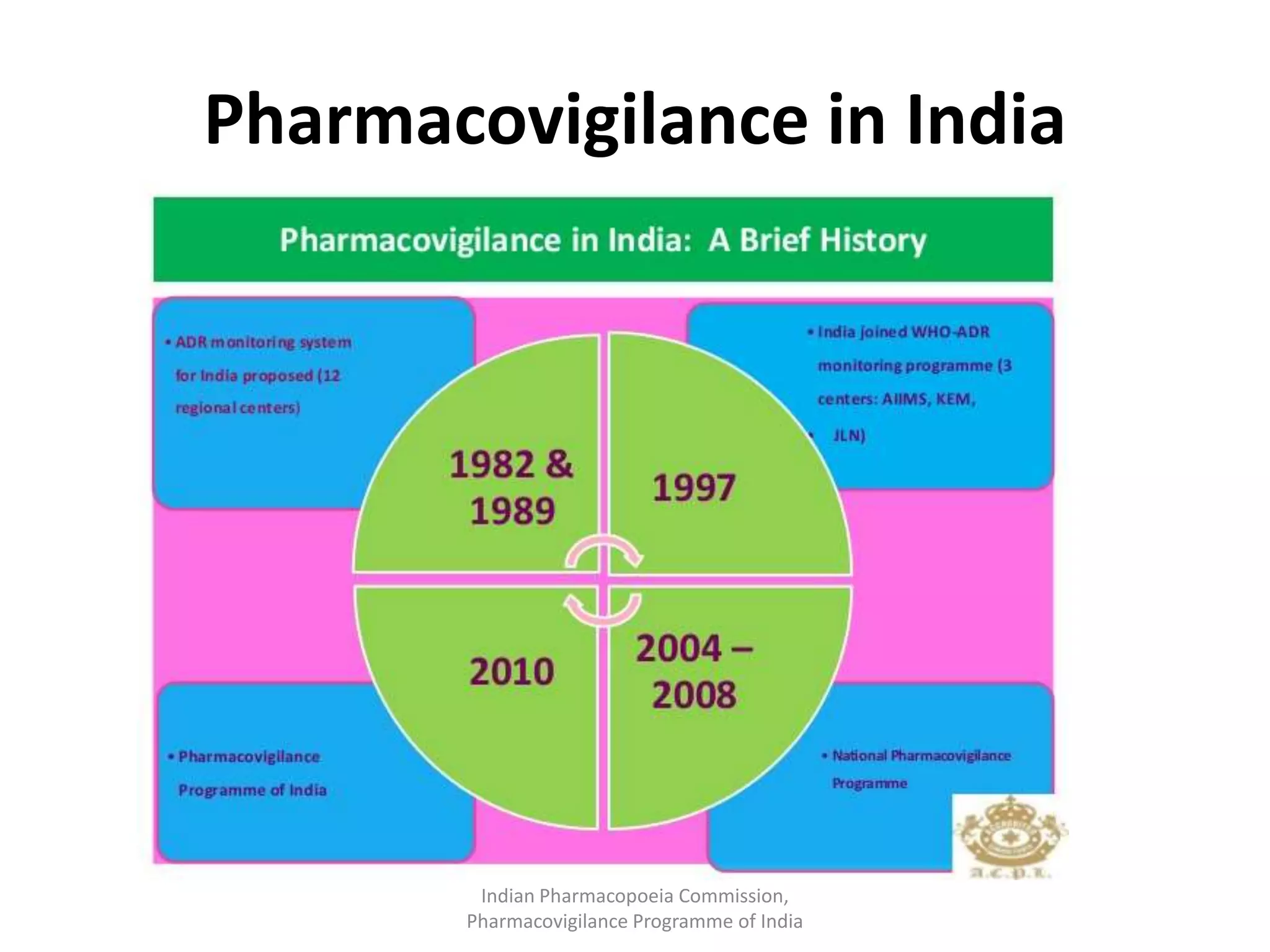
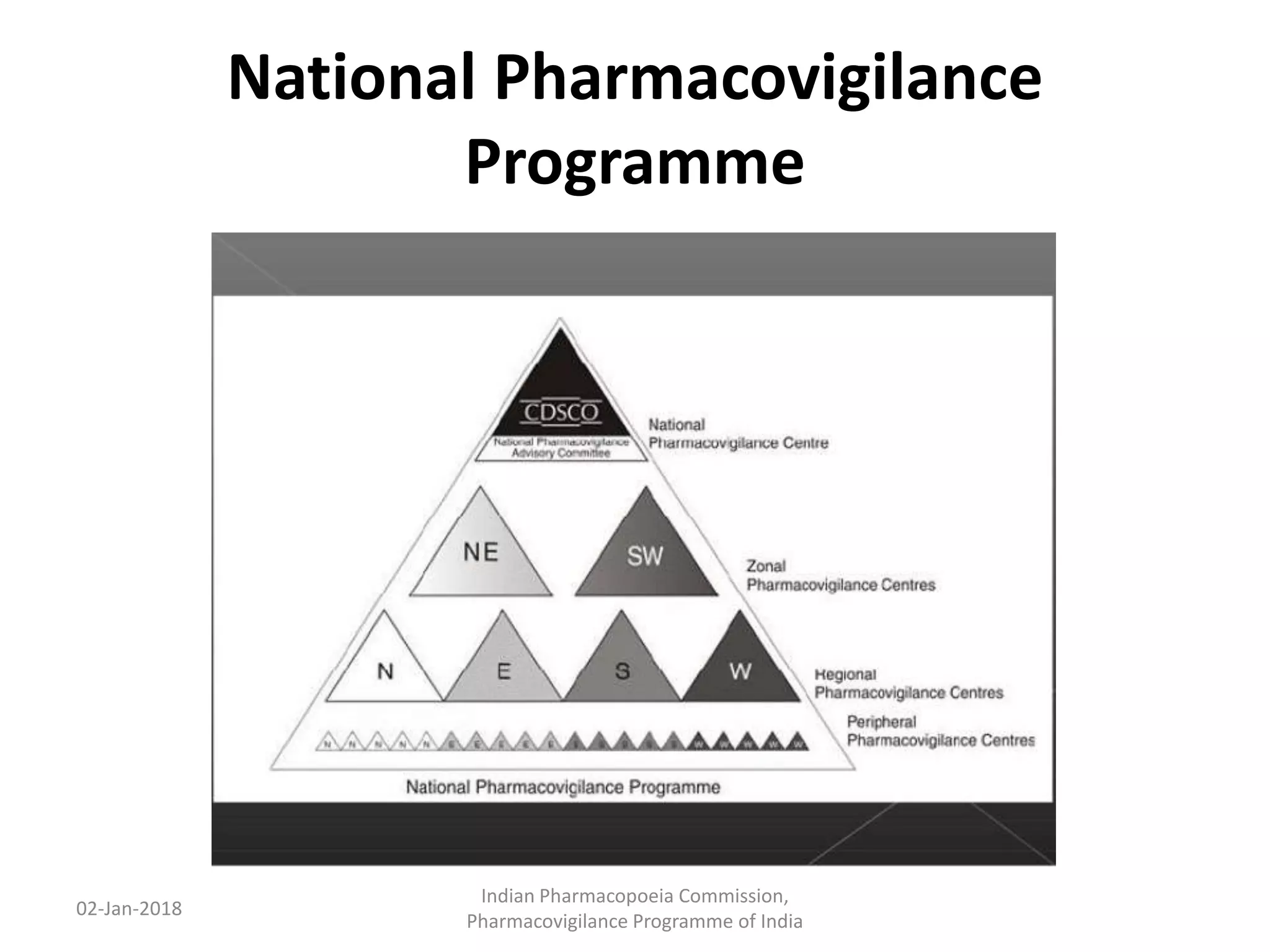
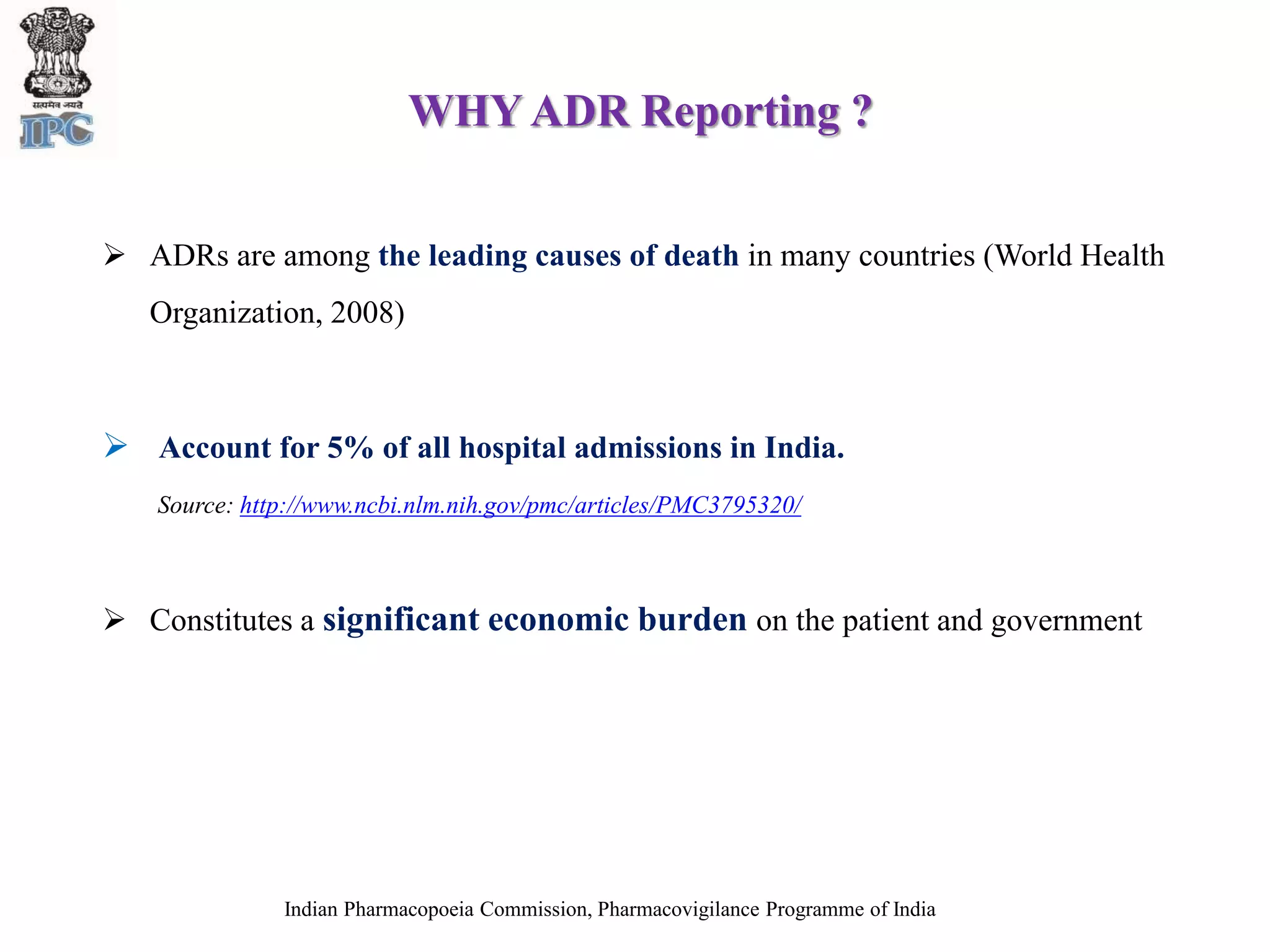
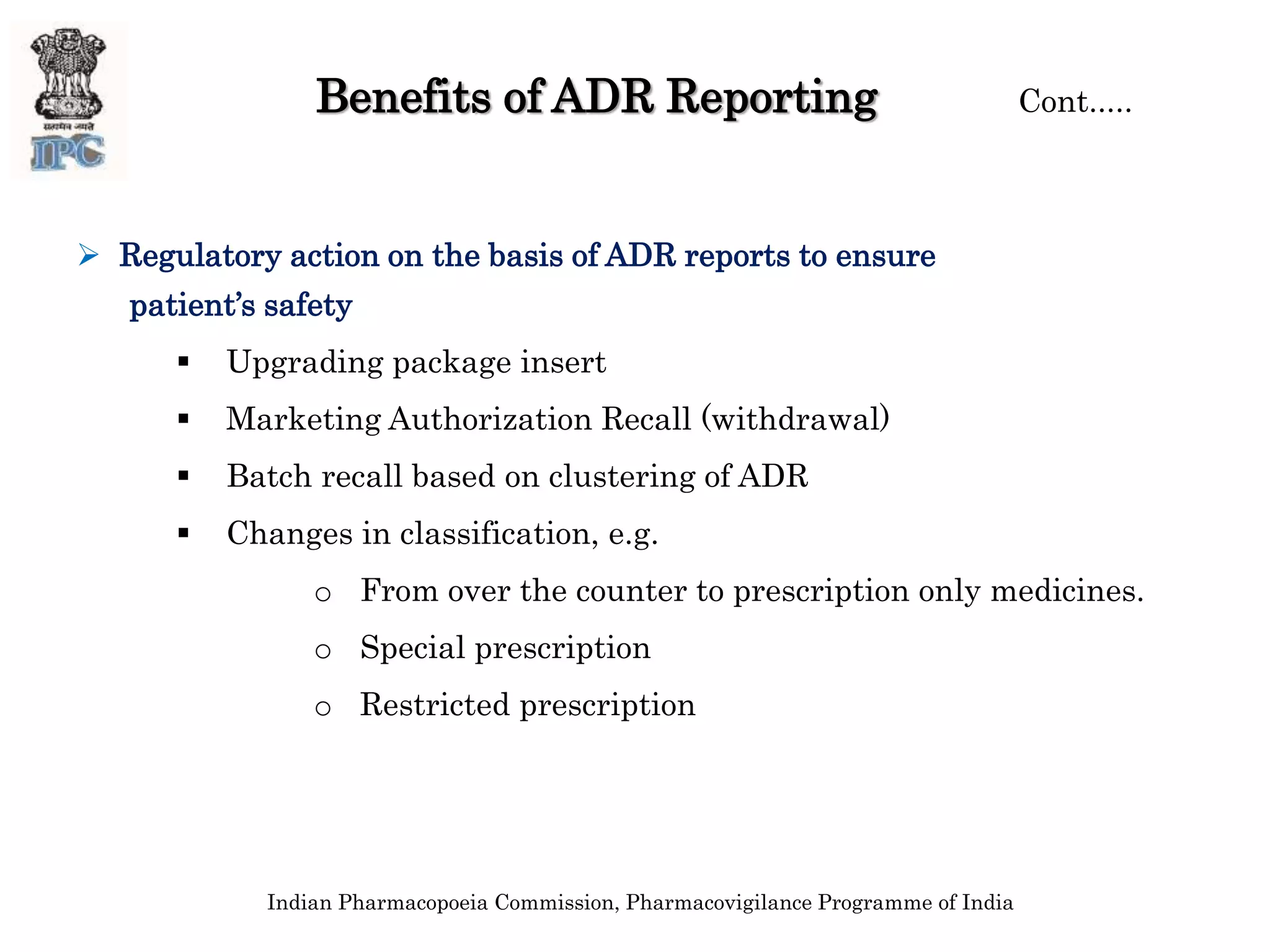
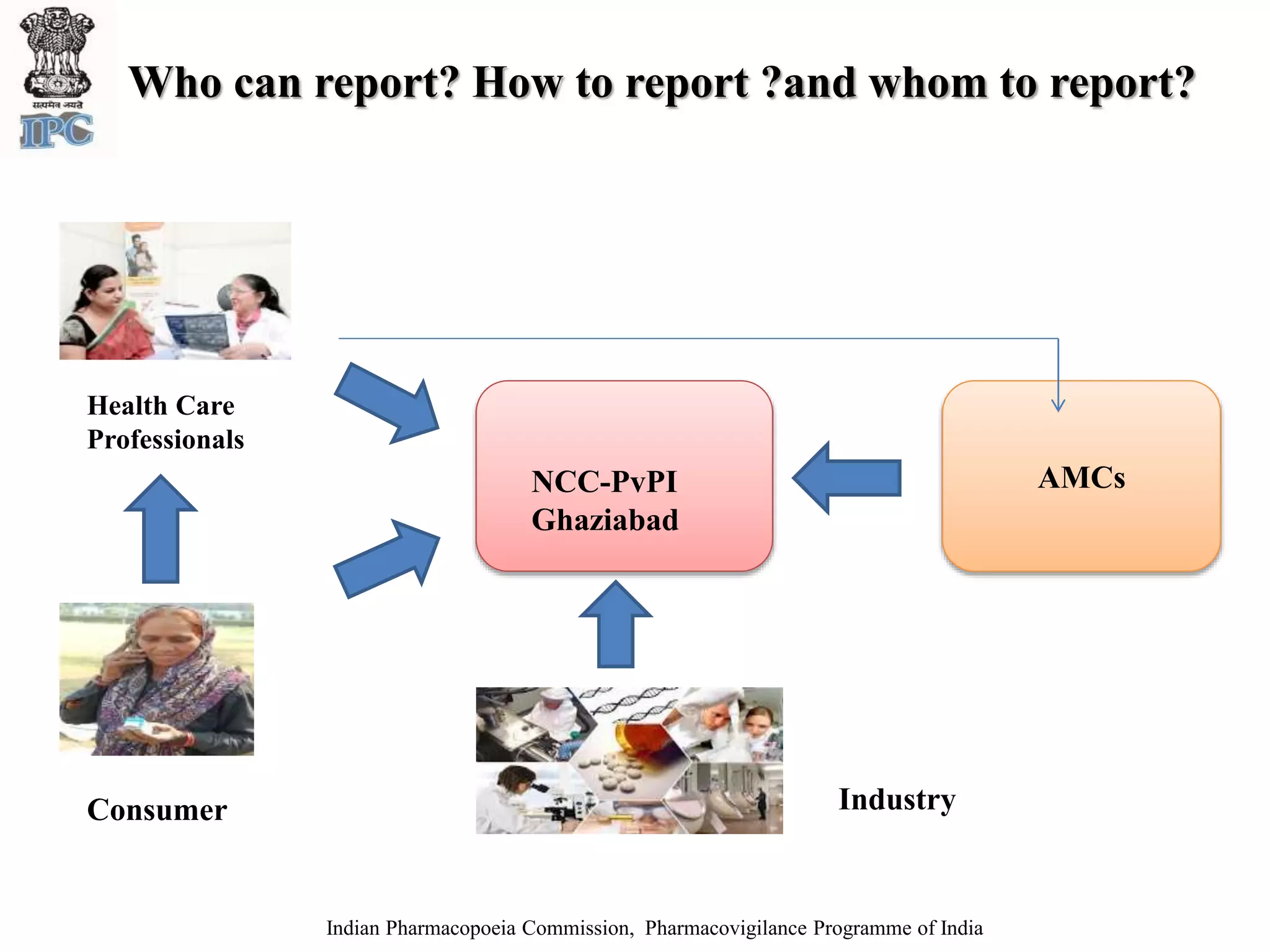
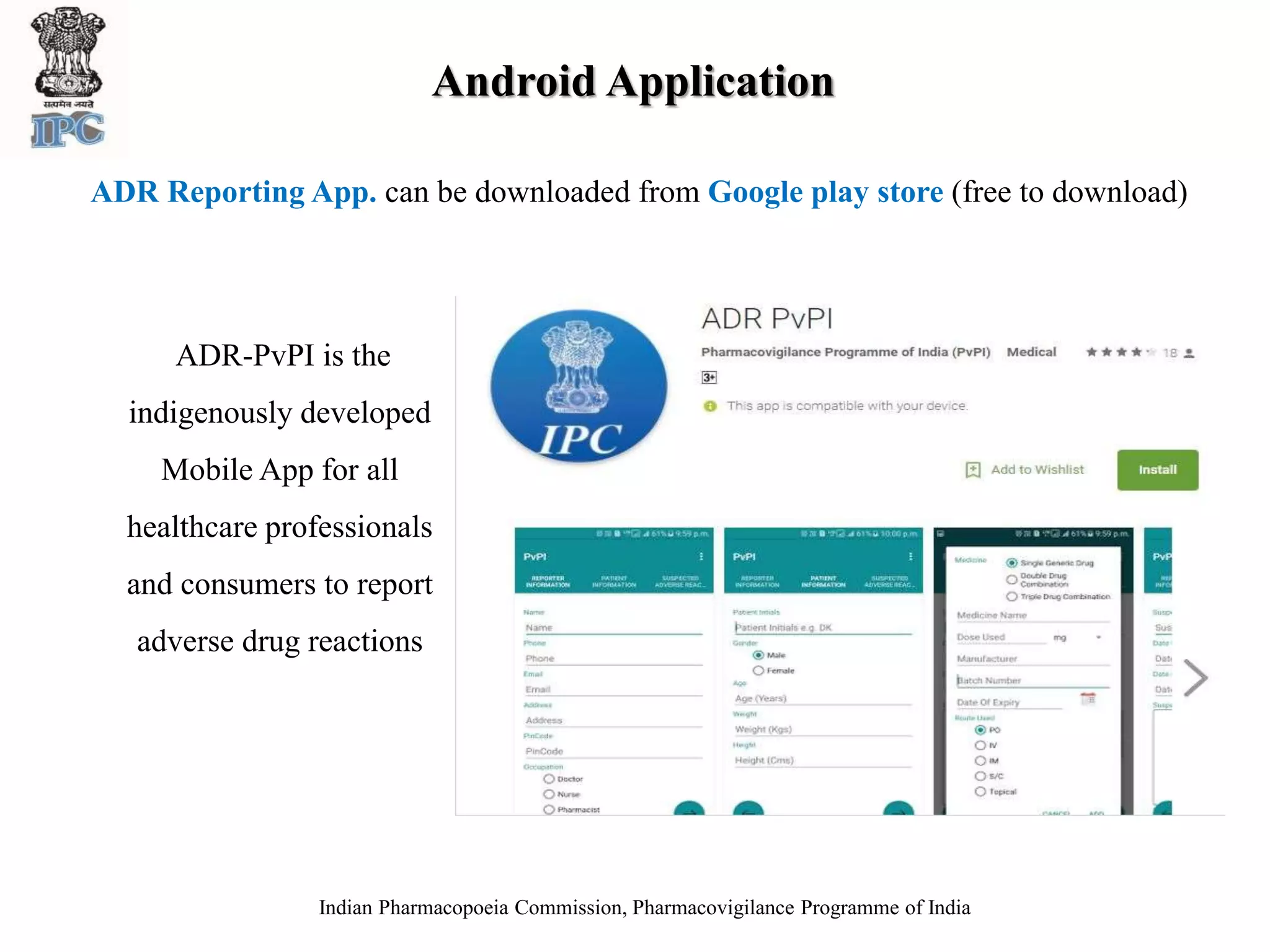
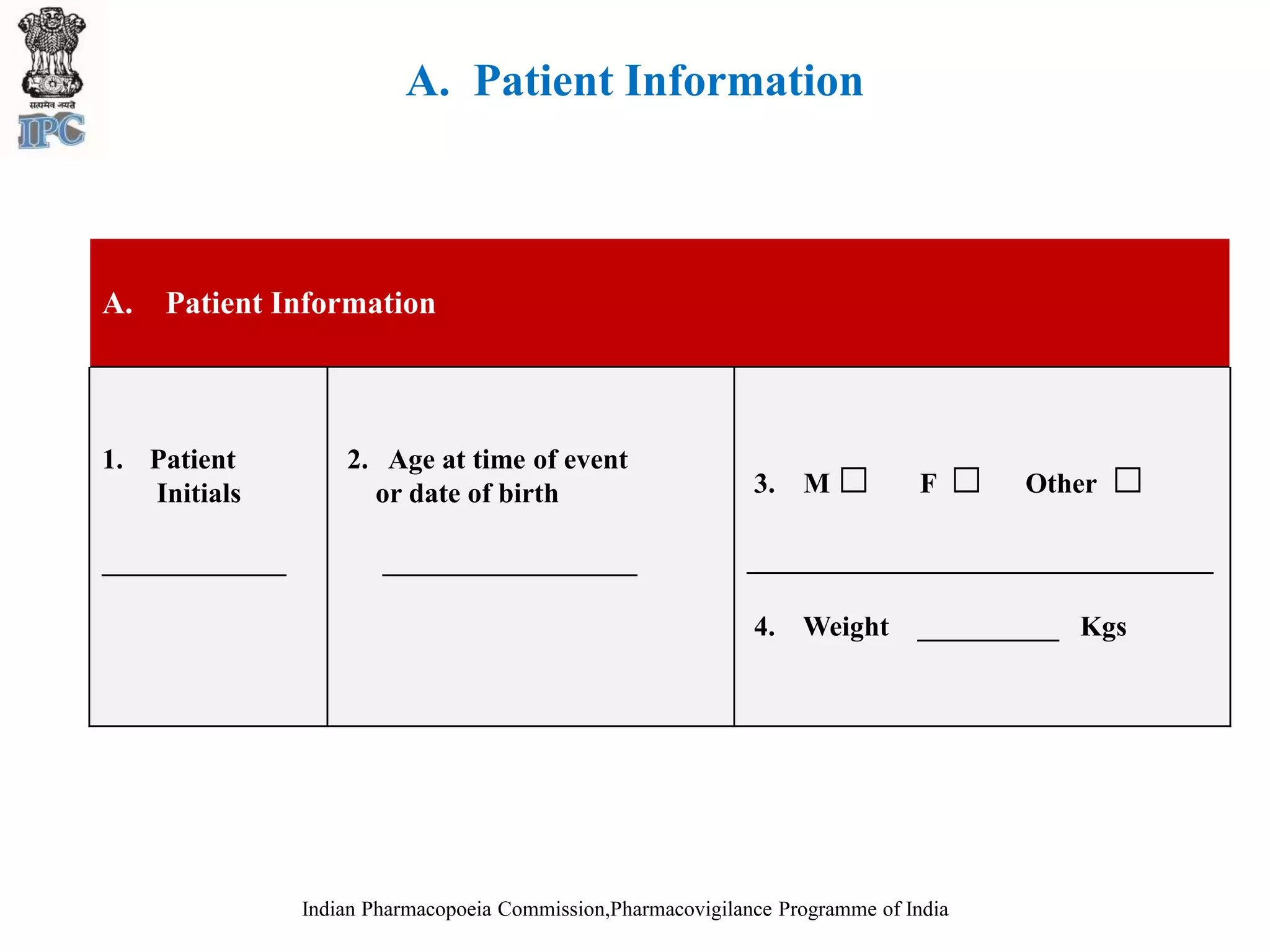
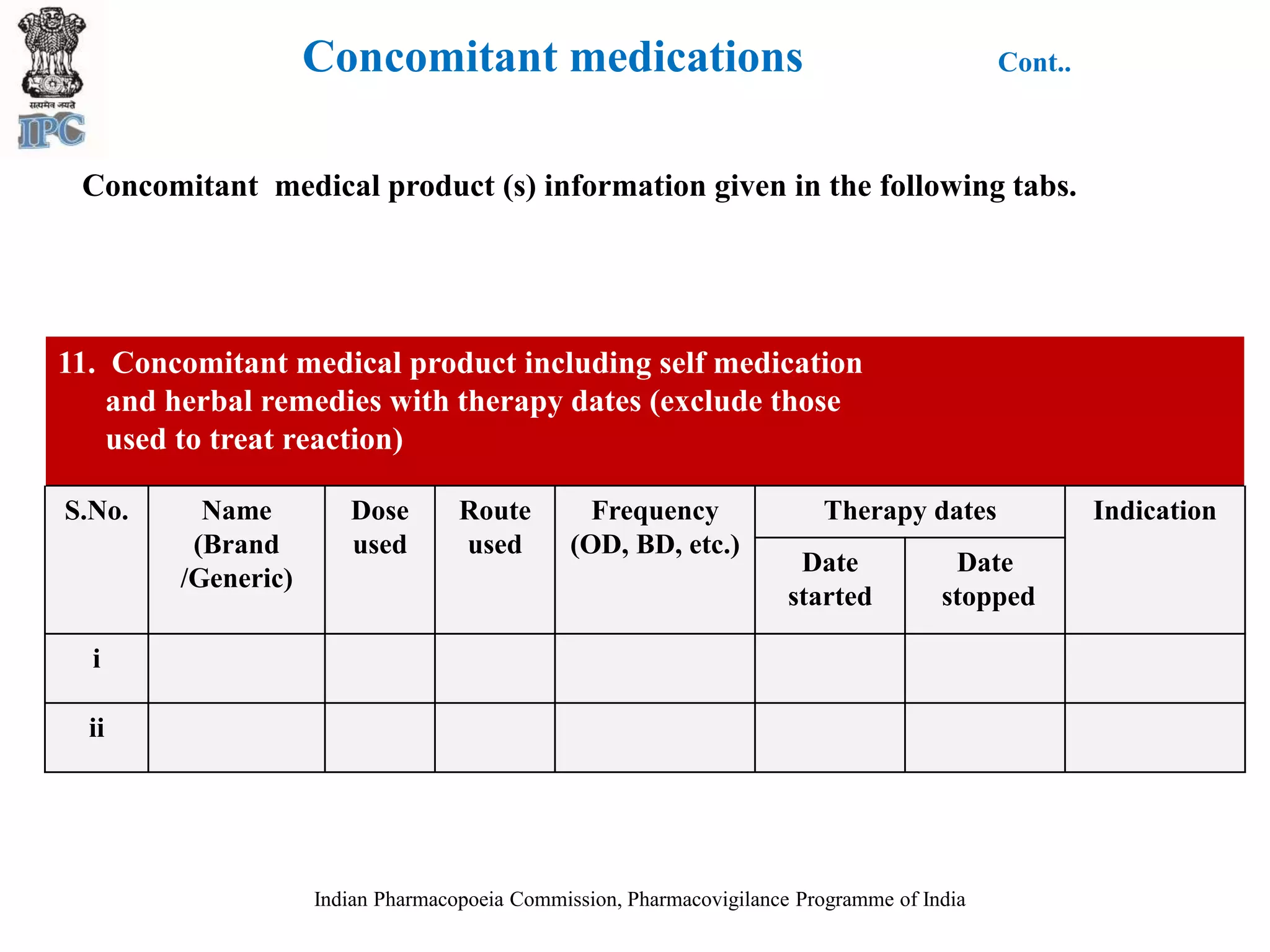
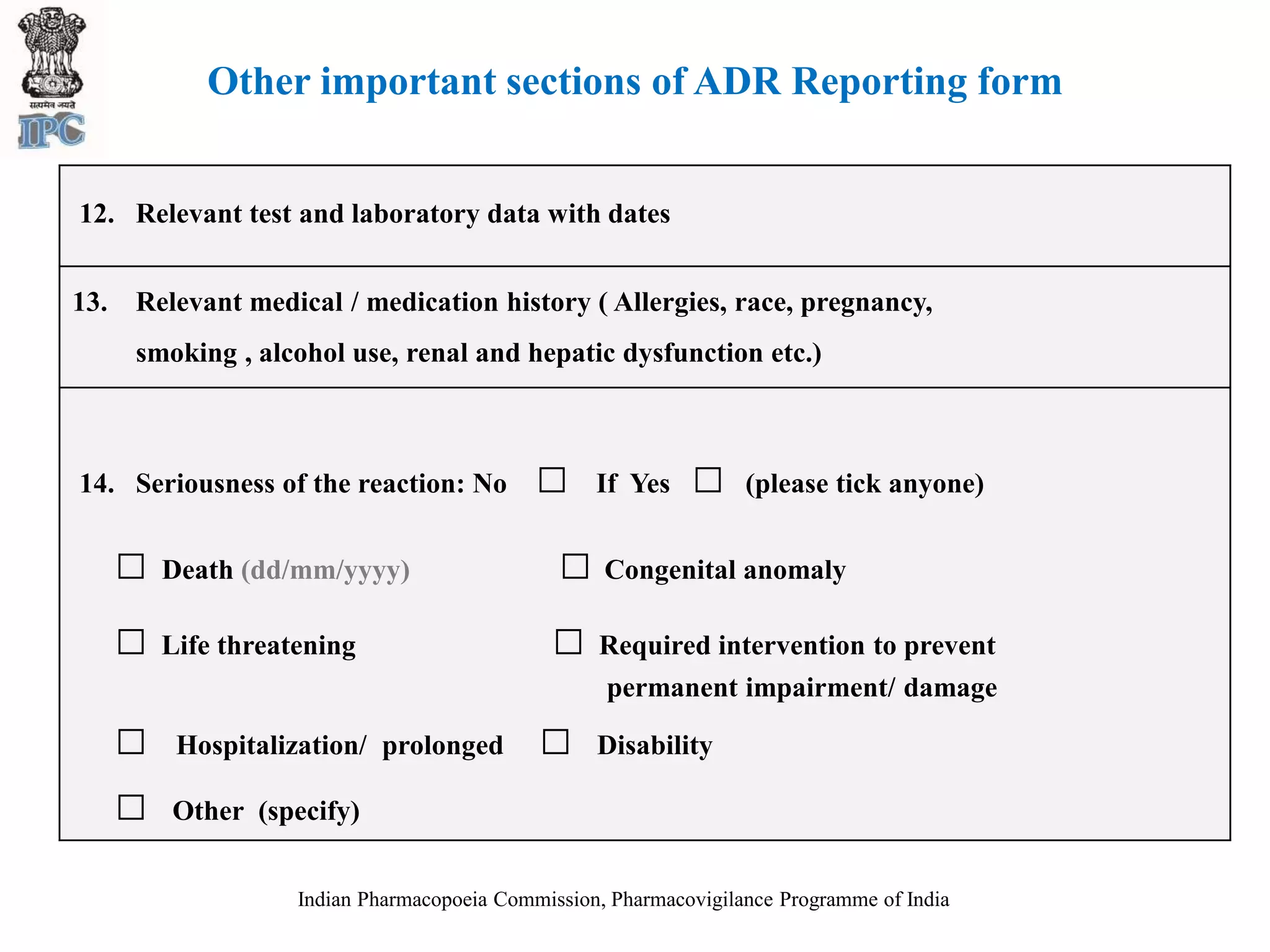
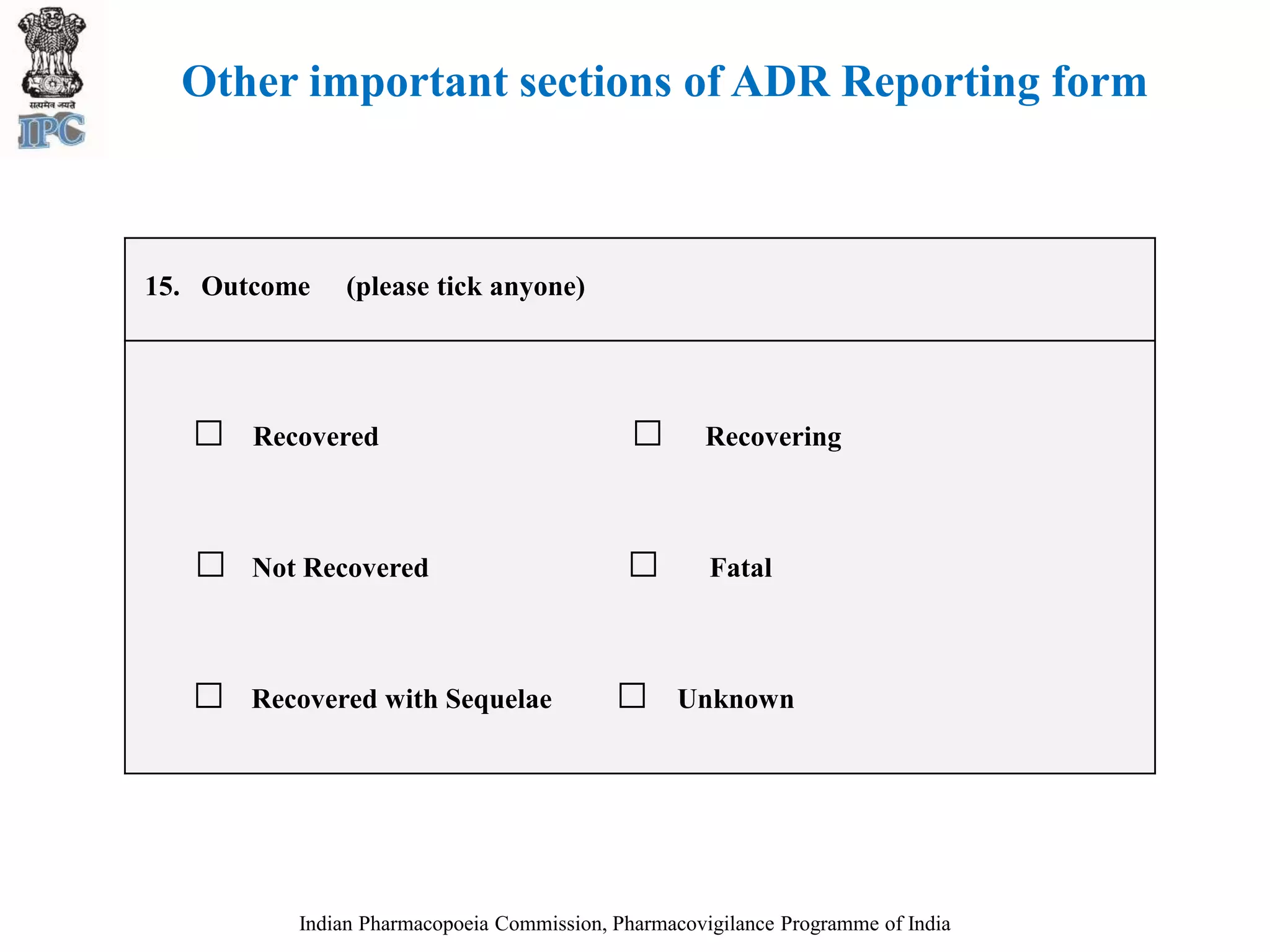
The document discusses pharmacovigilance and adverse drug reaction (ADR) reporting in India. It provides information on the national pharmacovigilance program, including who can report ADRs, how to report them, and the benefits of reporting. It describes the ADR reporting process and forms for healthcare professionals and consumers. It also discusses other vigilance programs in India related to medical devices, vaccines, blood products, and several research projects conducted with these programs.