The document outlines the evolution and implementation of the pharmacovigilance program in India, aimed at improving patient safety through comprehensive drug safety monitoring. It highlights key historical milestones, objectives, goals, and methods associated with the program, while also addressing challenges such as underfunding and low healthcare professional involvement. The National Coordination Centre (NCC) at IPC is tasked with establishing a nationwide framework to support reporting and analysis of adverse drug reactions (ADRs).

![Instruction
BACKGROUNG CHECK:
1989 – ADR monitoring system for India was
proposed [12 regions]
1997 – India joined WHO-ADR monitoring
programme [3 centers-AIIMS,KEM.JLN]
2004-2008 – National pharmacovigilance
programme
2010 – Pharmacovigilance programme of
India[PvPI]
As per WHO:
Pharmacovigilance is a system relating to
the detection,assessment,understanding
and prevention of adverse effect and any
other drug related problems.
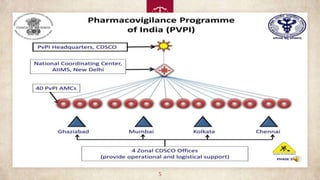
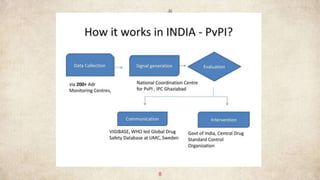
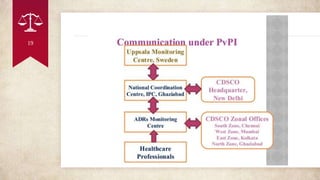
PvPI is a programme initiated with AIIMS New Delhi as National Coordination centre(NCC) for
monitoring ADR in the country 14th July 2010 shifted to India pharmacopoeia commission (IPC)
,Ghaziabad, Uttar Pradesh on 15th April 2011.
2](https://image.slidesharecdn.com/pharmacovigilance-220526121356-6be49990/85/pharmacovigilance-program-in-india-pptx-2-320.jpg)



















![Referance
Google
oArticle by –innovations in pharmacy
25
http://z.umn.edu/INNOVATIONS
oPPT by chandan kumar [Mpharm-
NIPER]
oPPT by Jamshed Ahmad –Sr.technical
data associate at CDISCO](https://image.slidesharecdn.com/pharmacovigilance-220526121356-6be49990/85/pharmacovigilance-program-in-india-pptx-25-320.jpg)