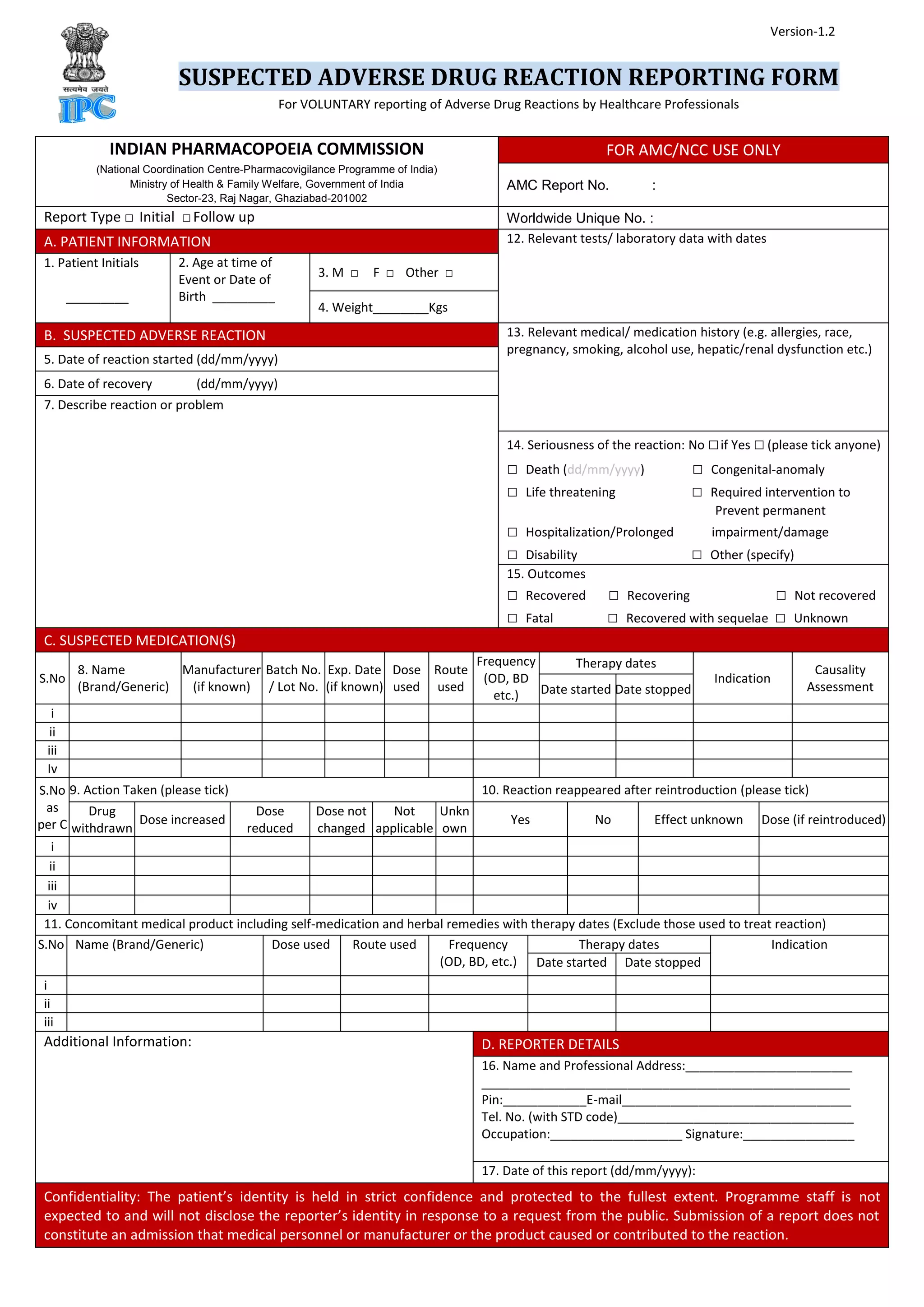
This document contains a suspected adverse drug reaction reporting form used by healthcare professionals in India to voluntarily report adverse reactions to medications. The form collects information about the patient, suspected reaction, suspected medications, actions taken, outcomes, and reporter details. It also provides instructions on what reactions to report, who can report, where to submit reports, how submitted information will be handled confidentially and analyzed to assess medication safety, and mandatory fields required for reporting.