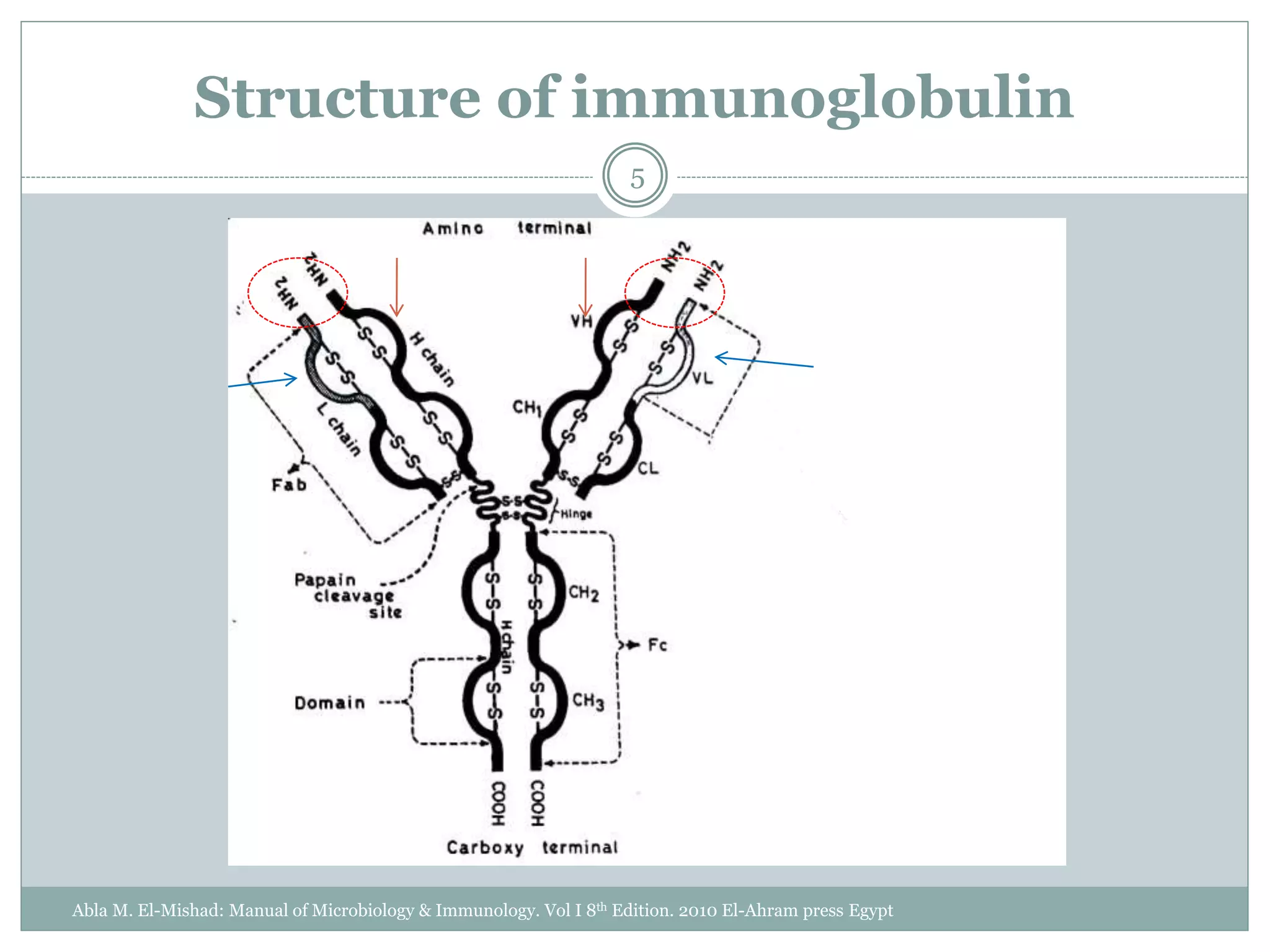
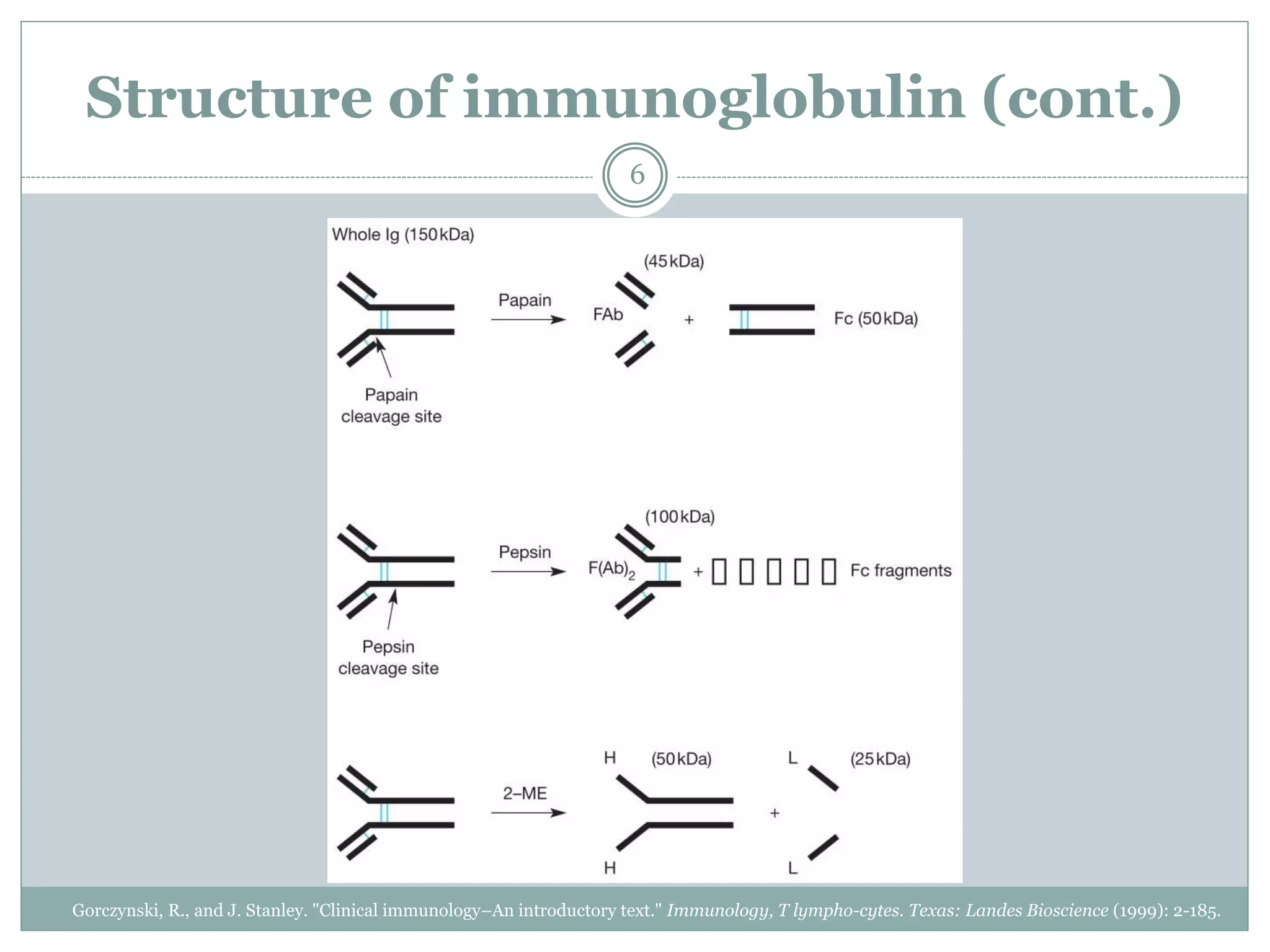
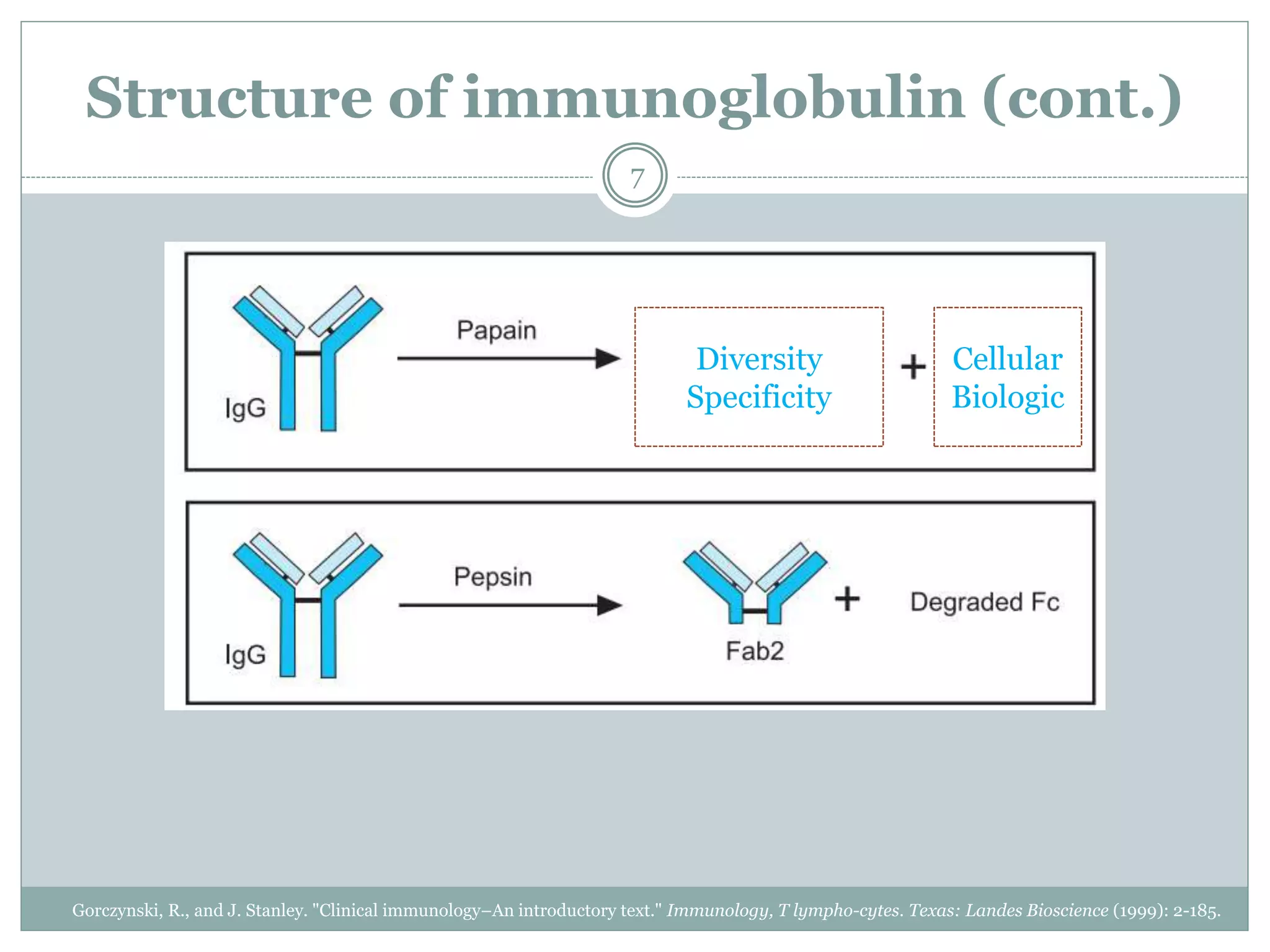
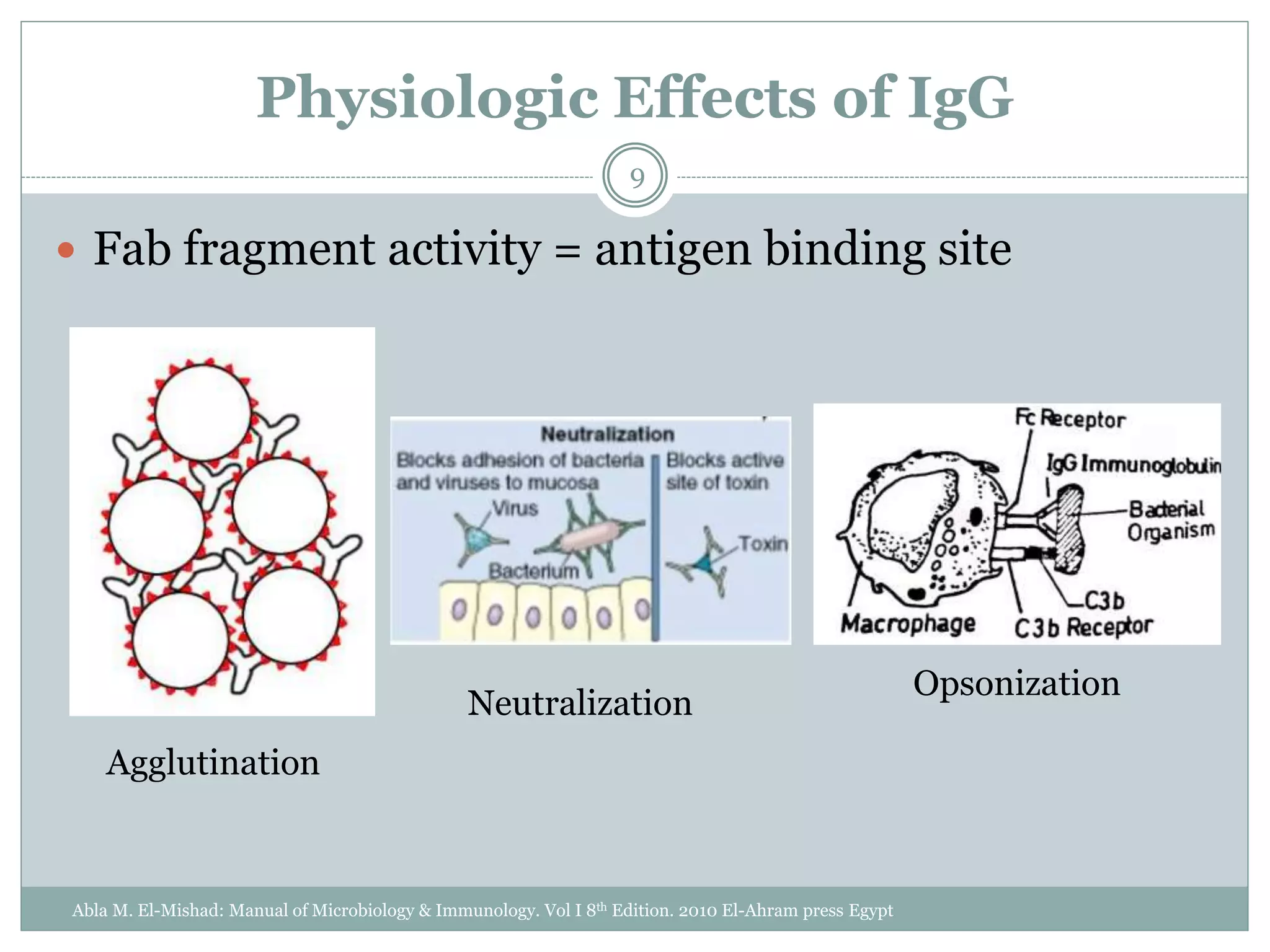
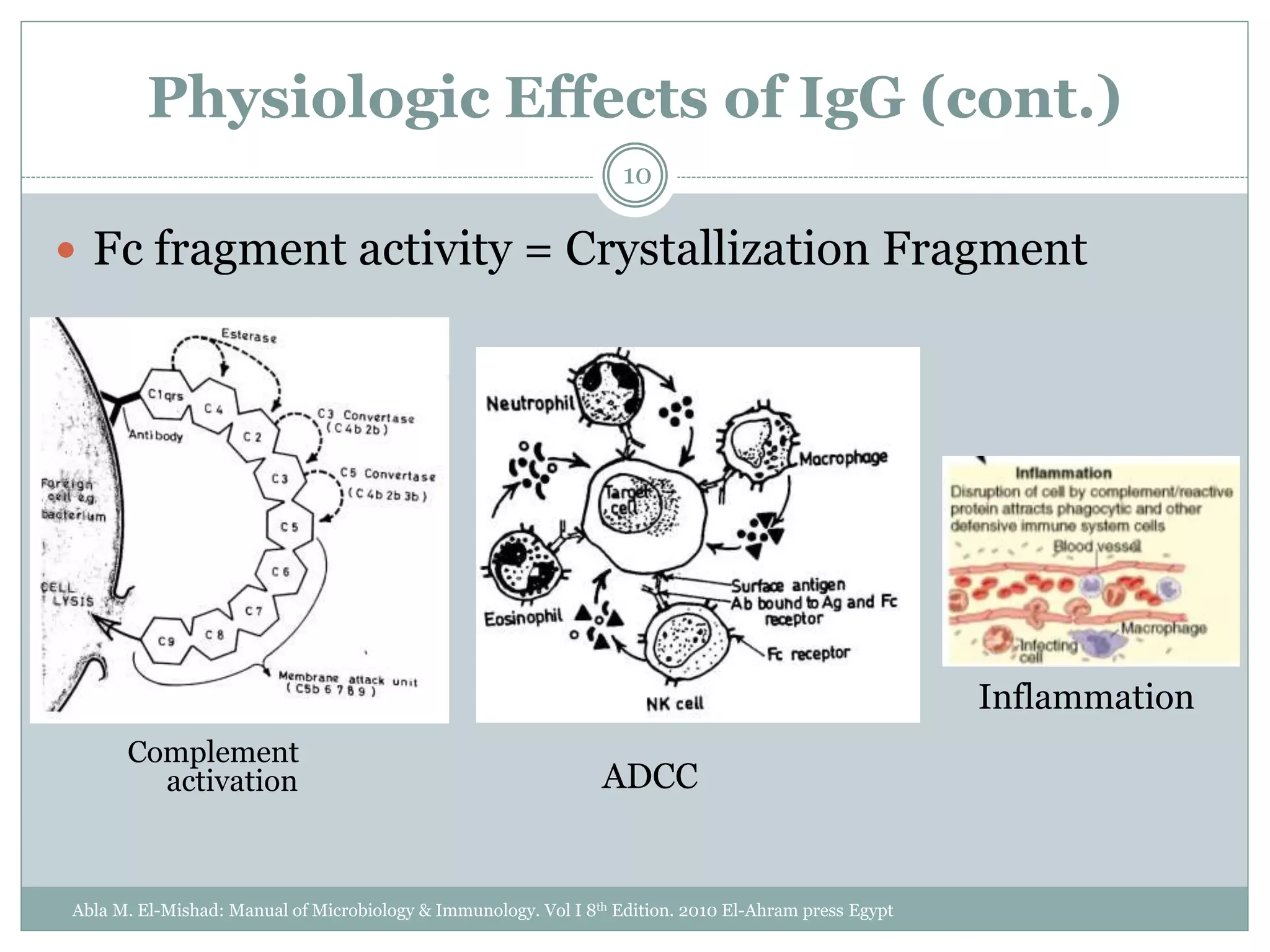
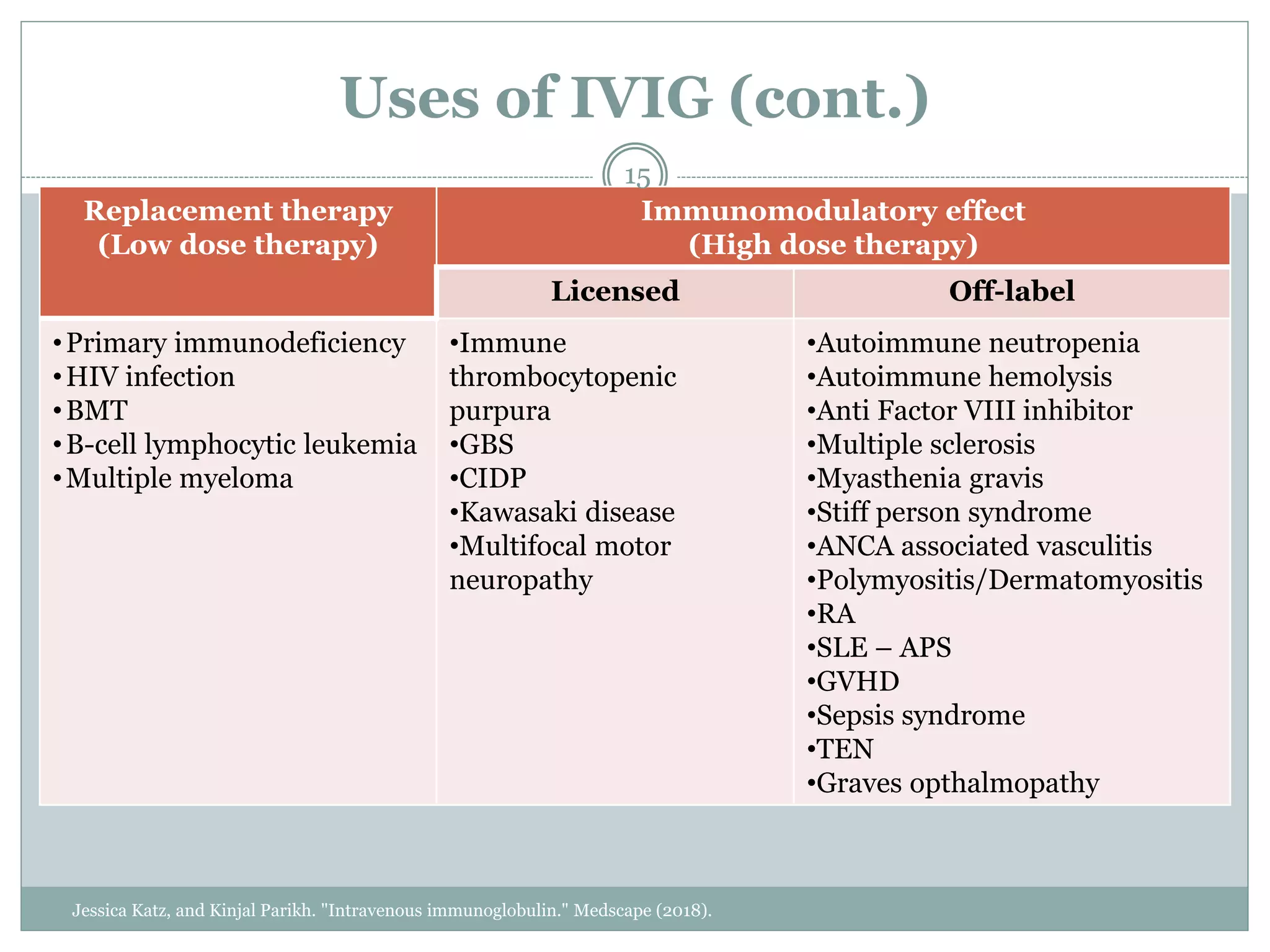
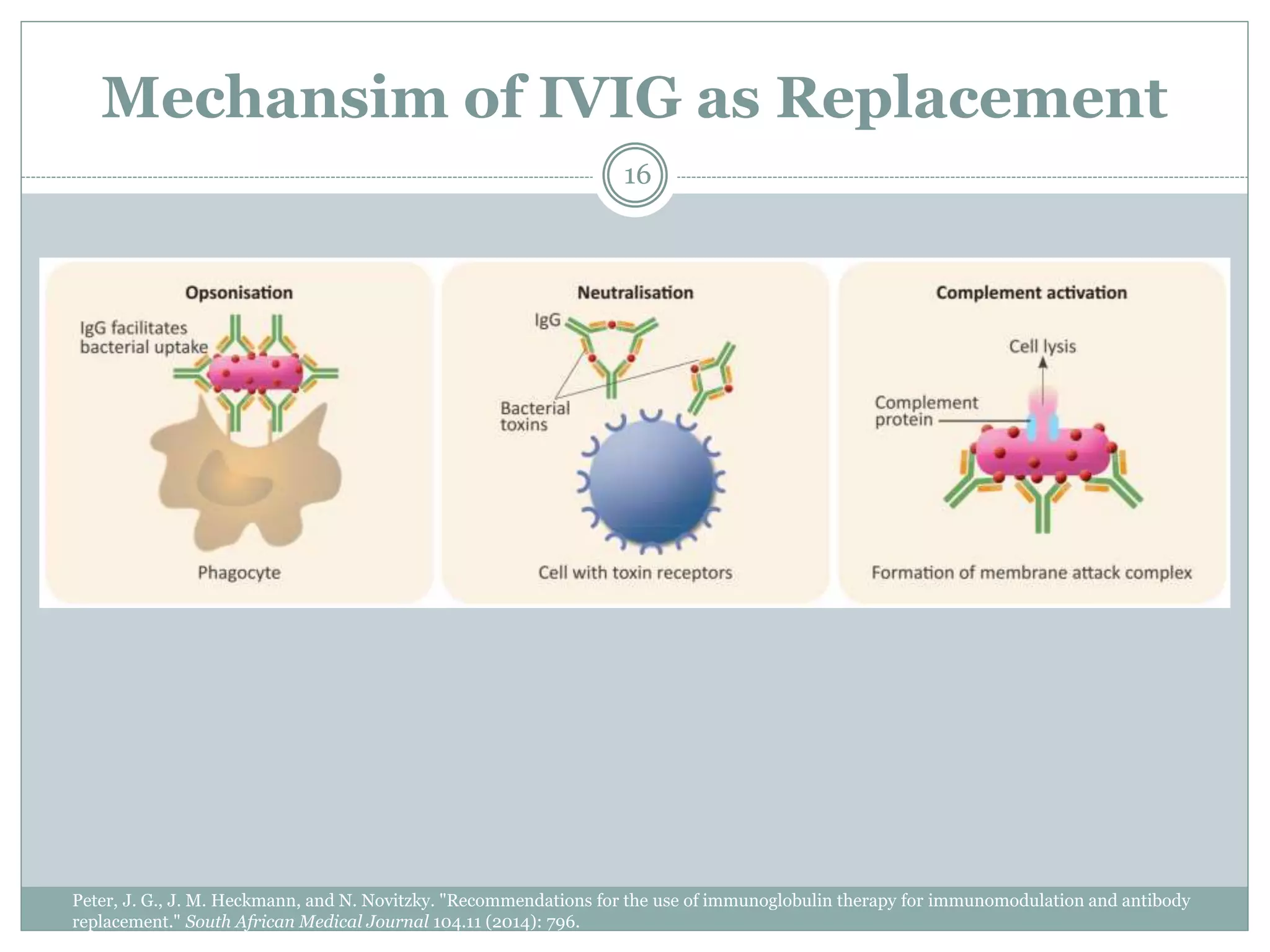
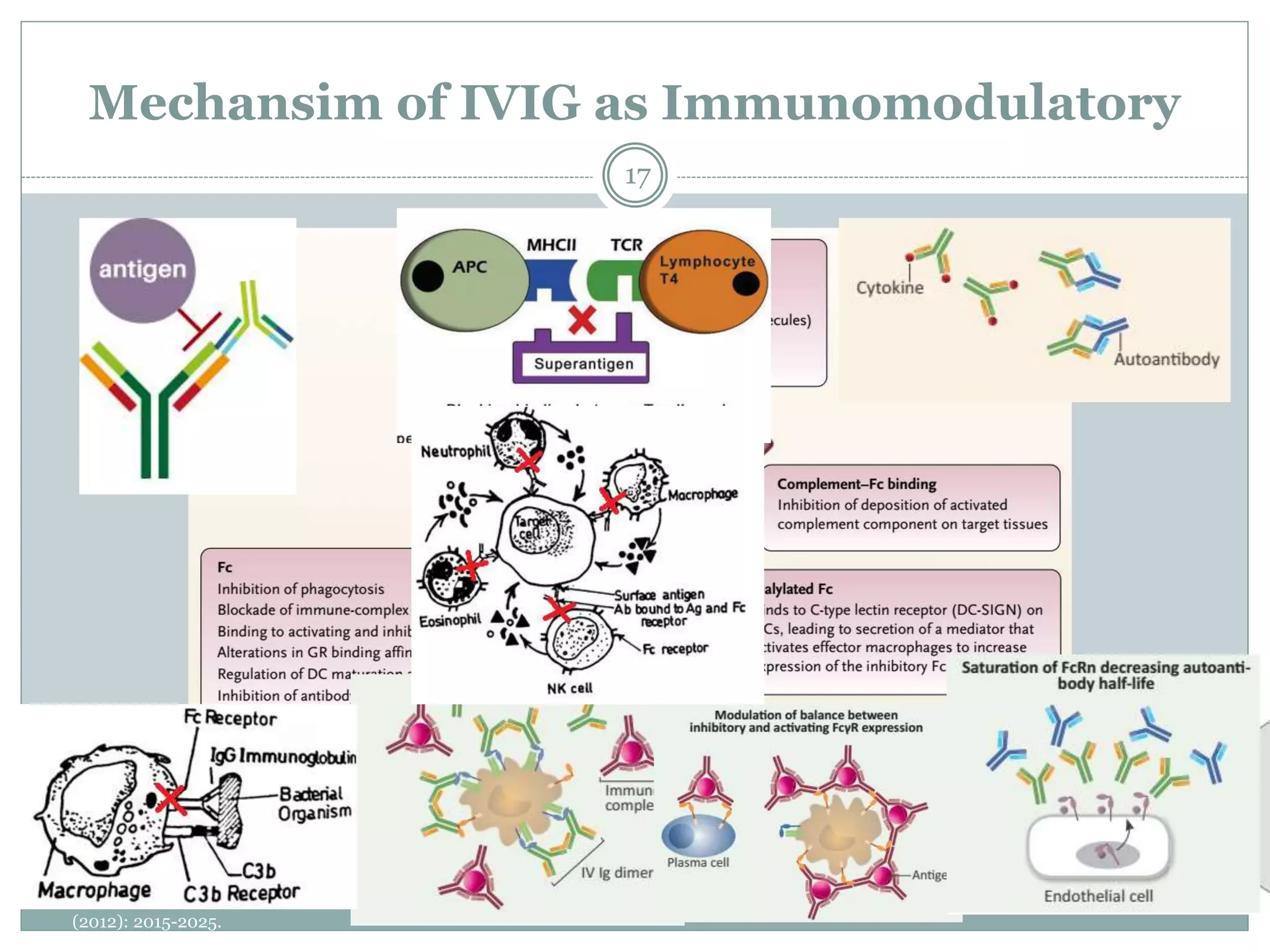
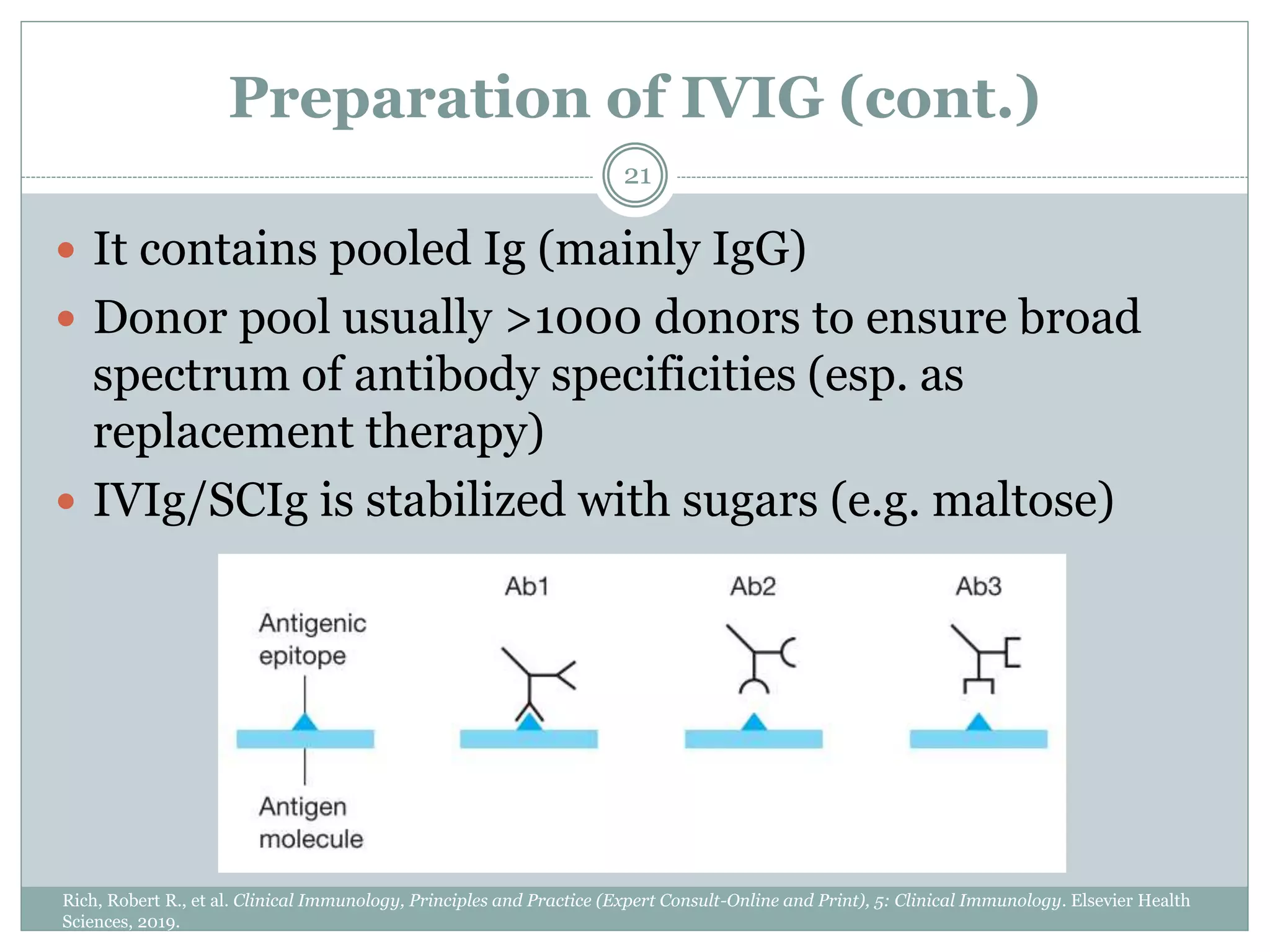
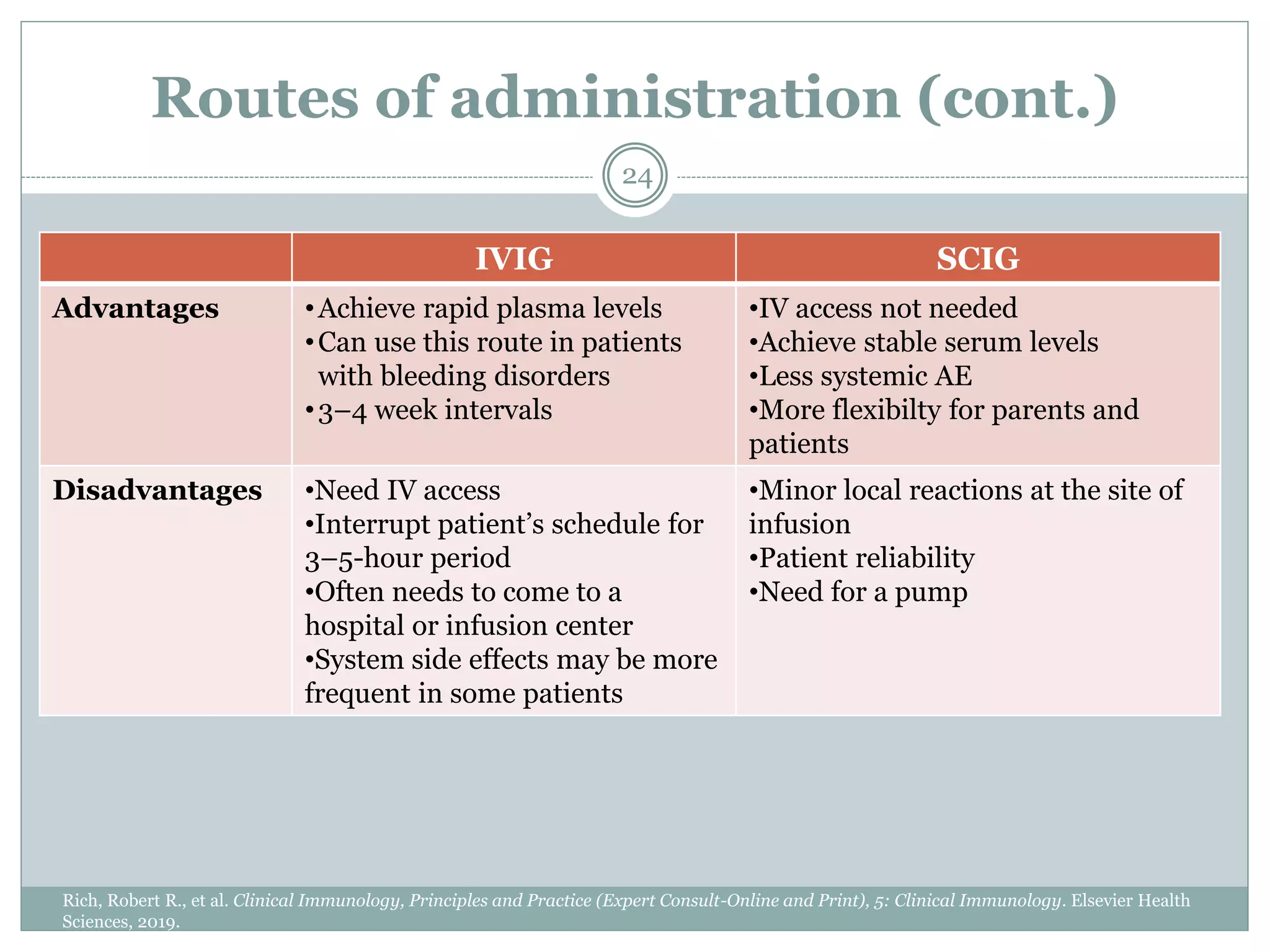
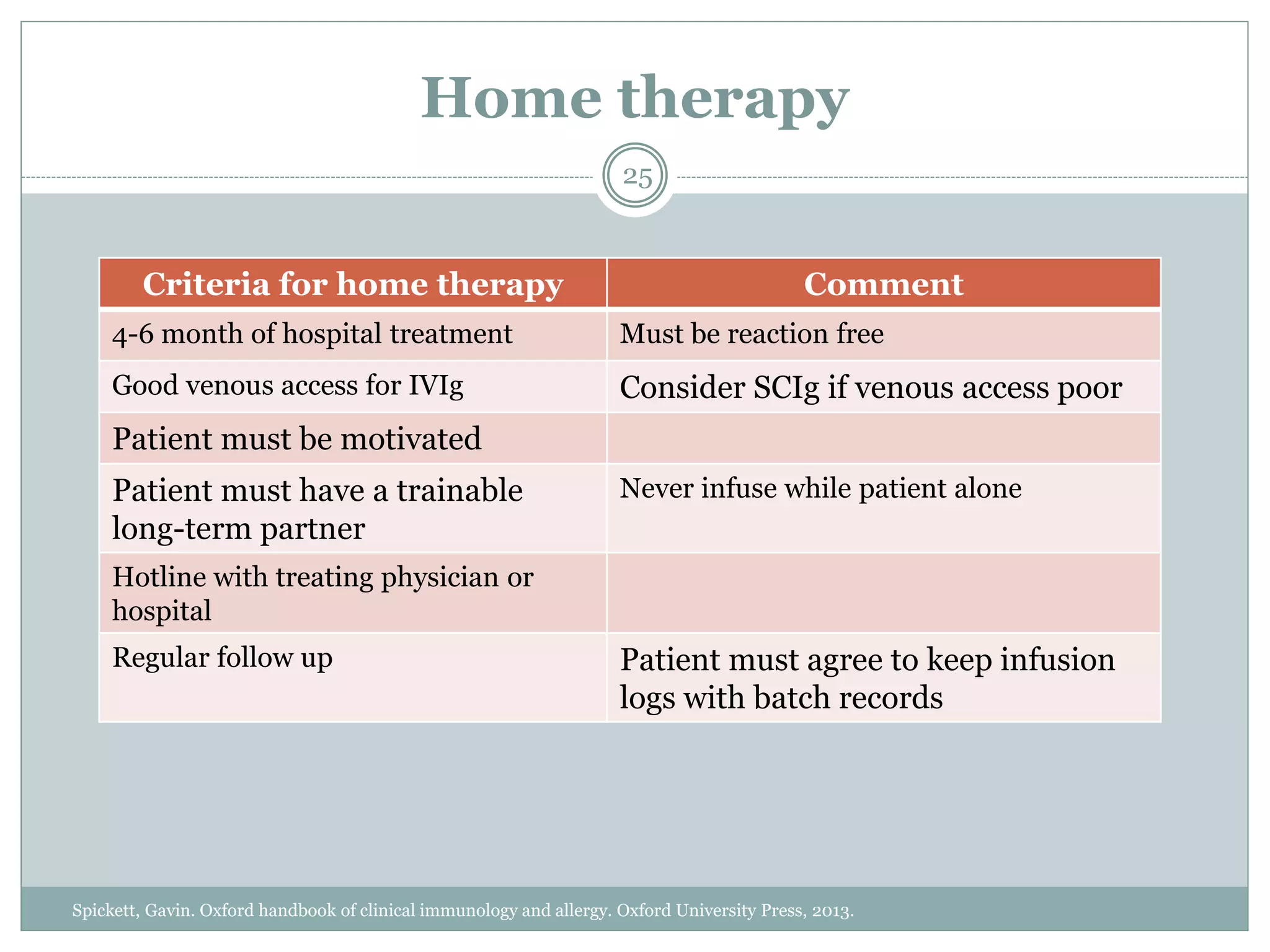
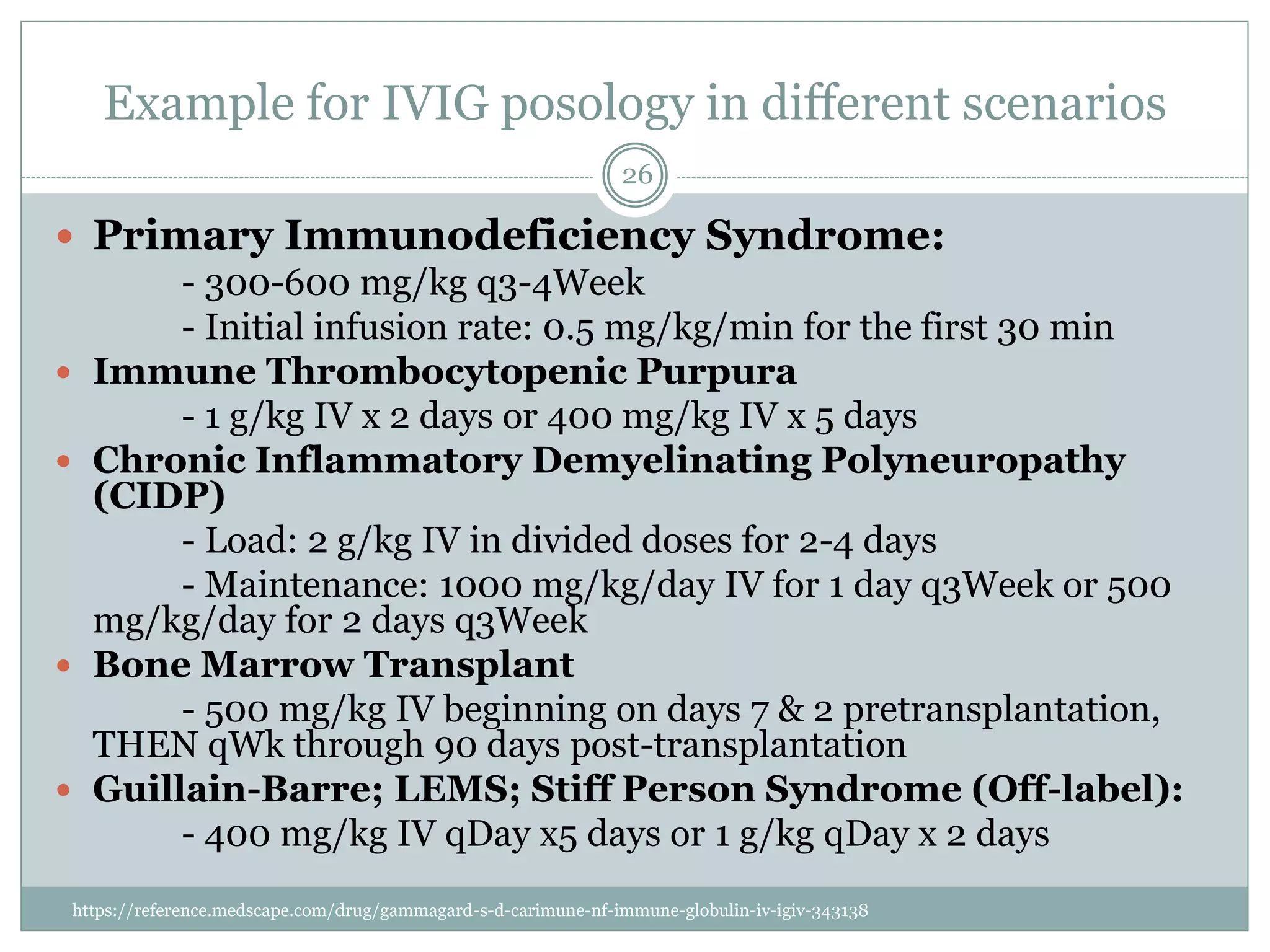
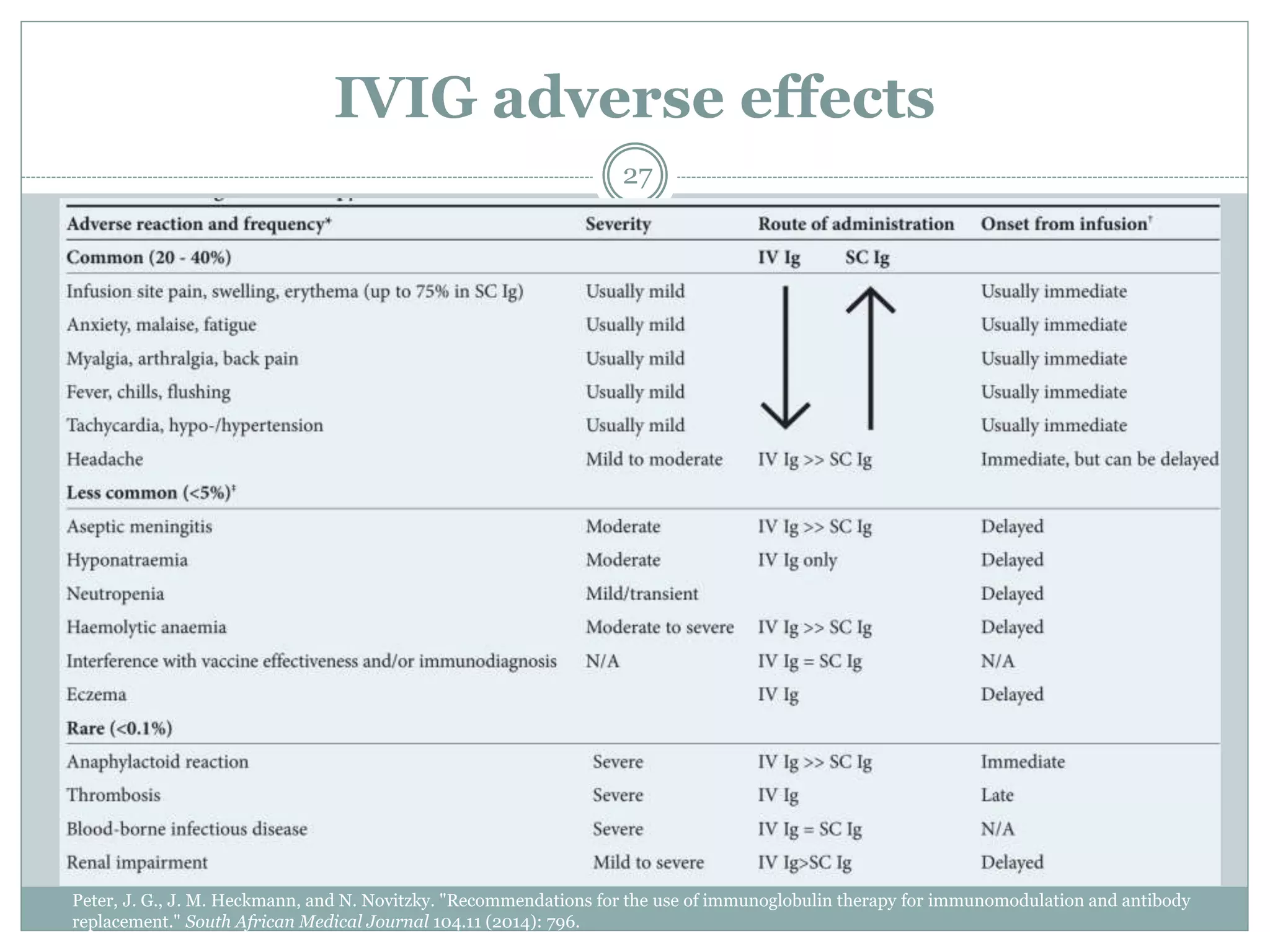
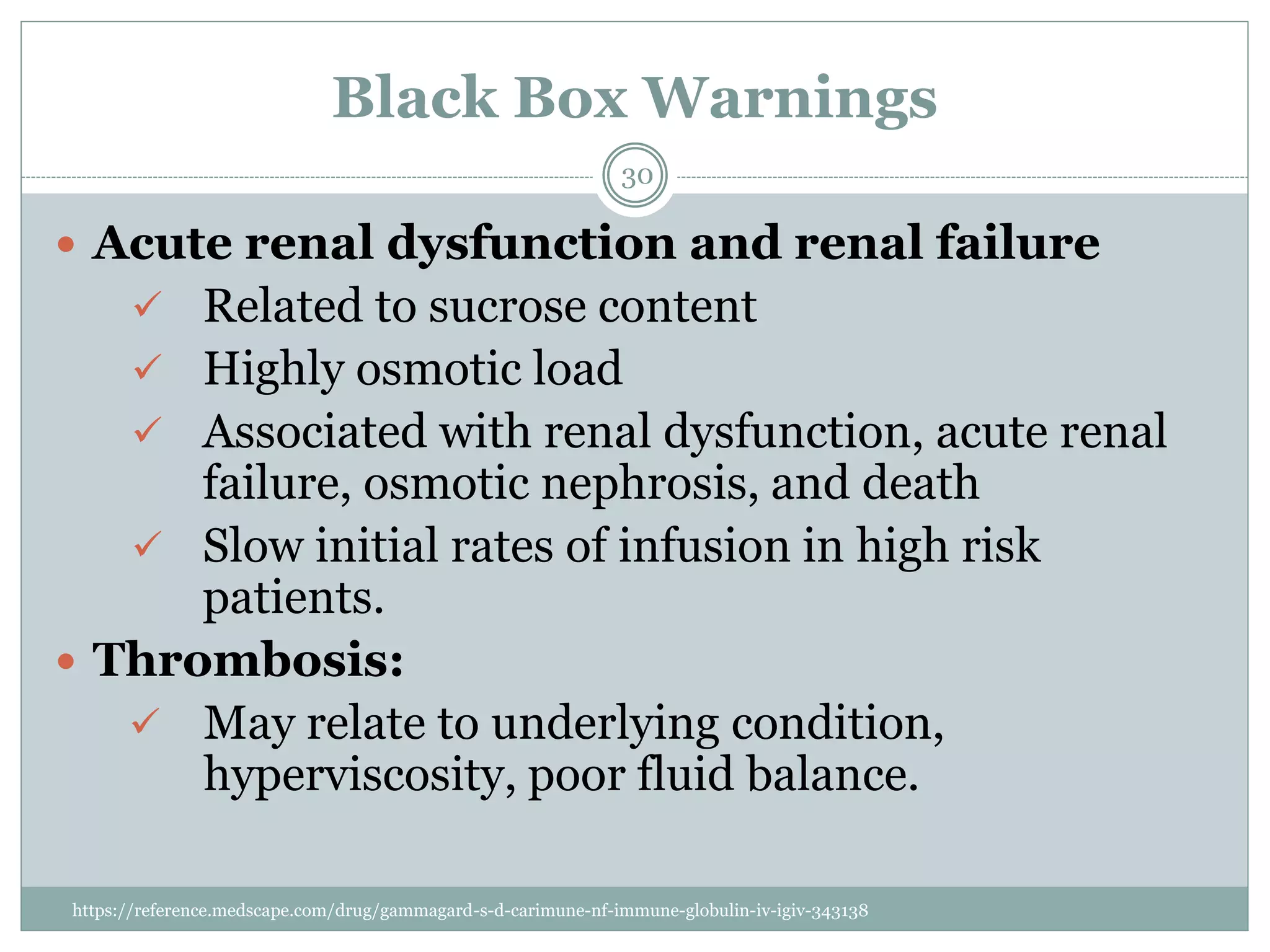
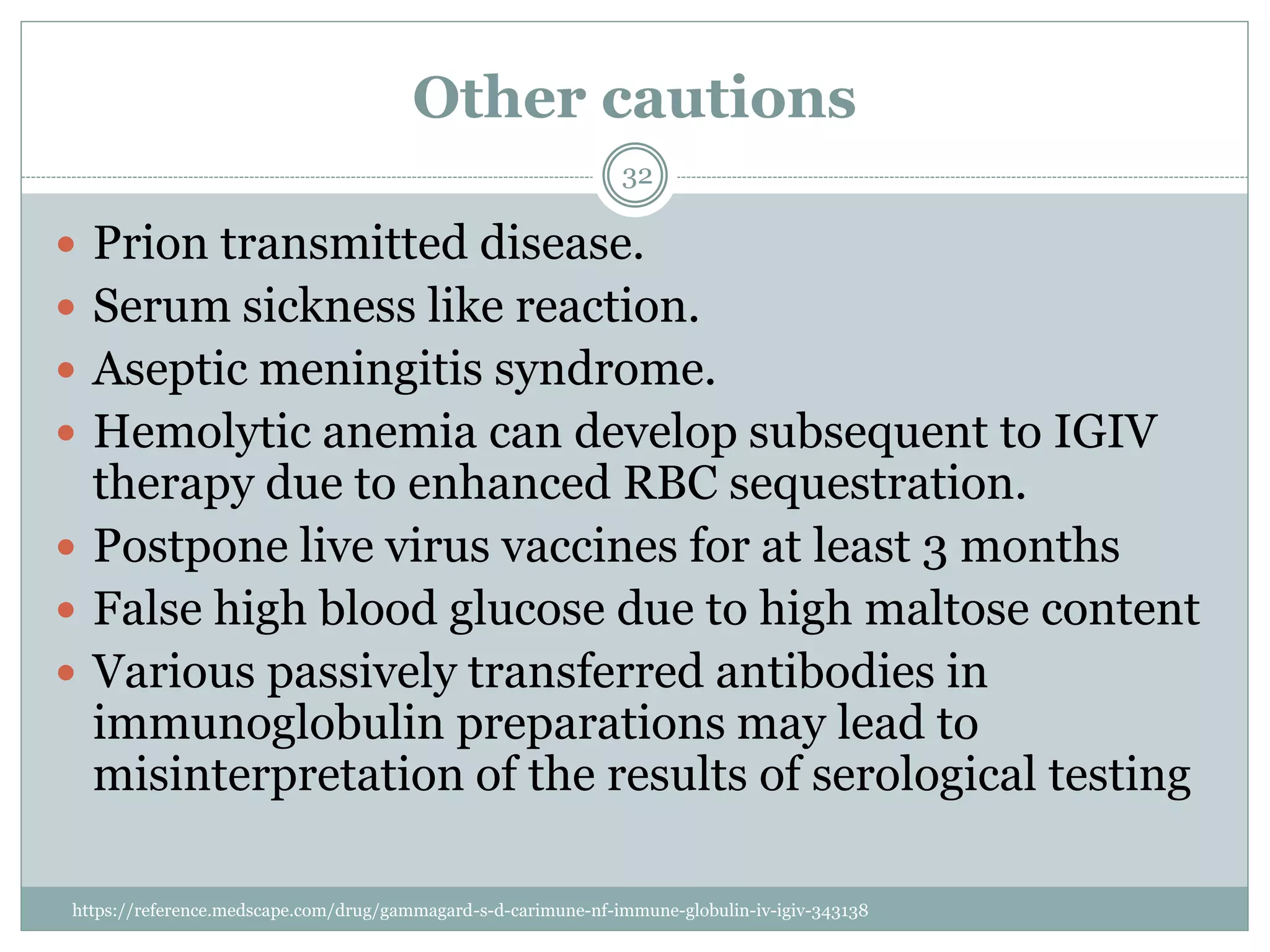
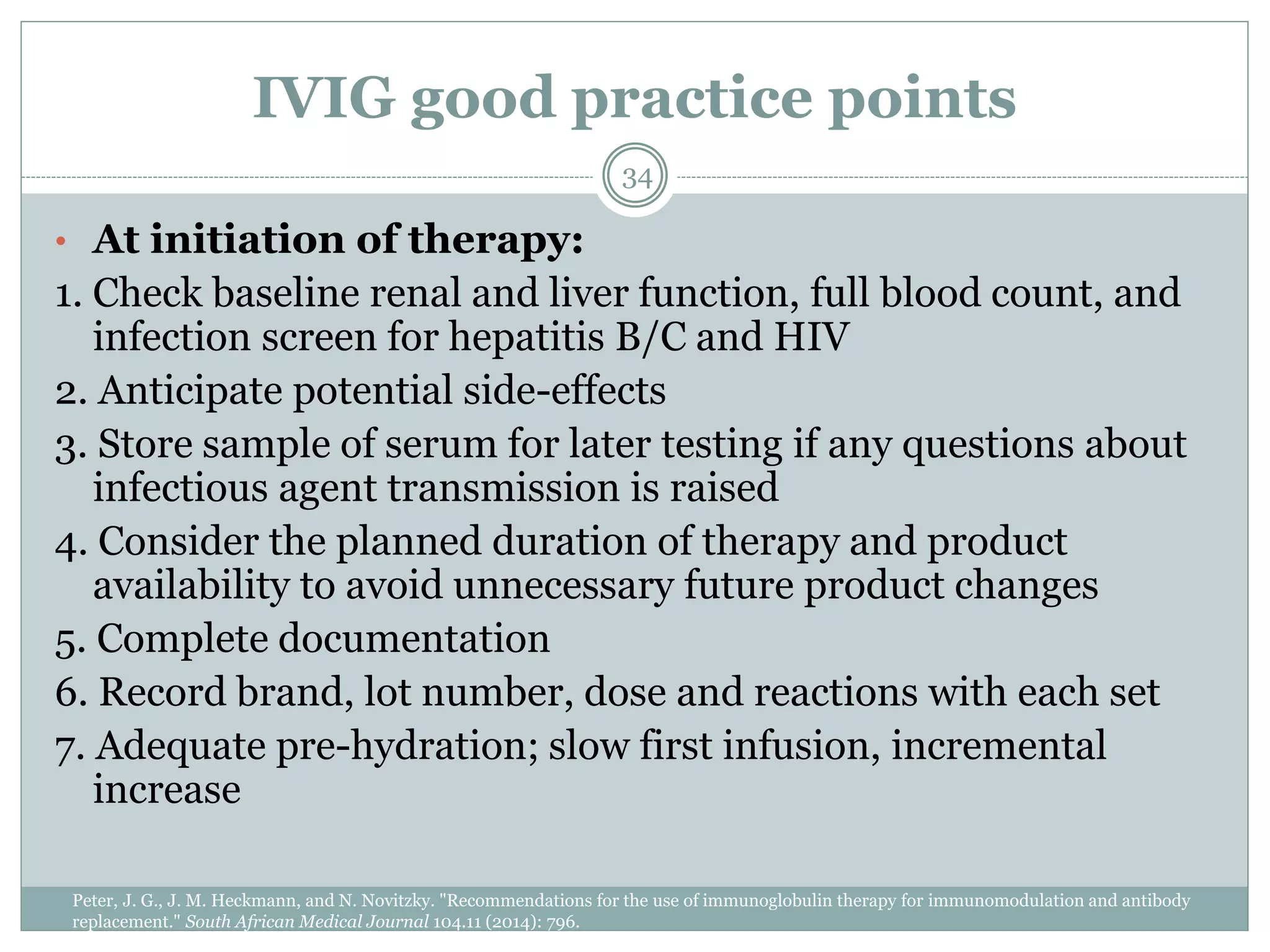
The document outlines the key aspects of intravenous immunoglobulin (IVIG) therapy, including its definitions, mechanisms of action, uses, and potential adverse effects. It emphasizes the importance of IVIG as a replacement therapy for various immunodeficiency disorders and its role in autoimmune diseases. Proper administration practices, contraindications, and good practice points for IVIG therapy are also discussed to ensure patient safety and efficacy.