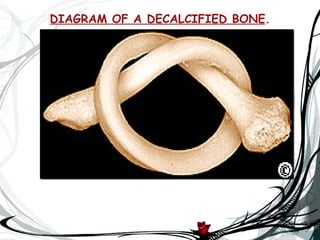
The document discusses bone decalcification, which is the process of removing calcium ions to make bones flexible for pathological investigation. It covers methods such as acid decalcifying agents, traditional methods, ion exchange resins, electrolytic decalcification, and chelating agents like EDTA. The document also addresses the purpose of decalcification, principles of the process, and tests for completion.