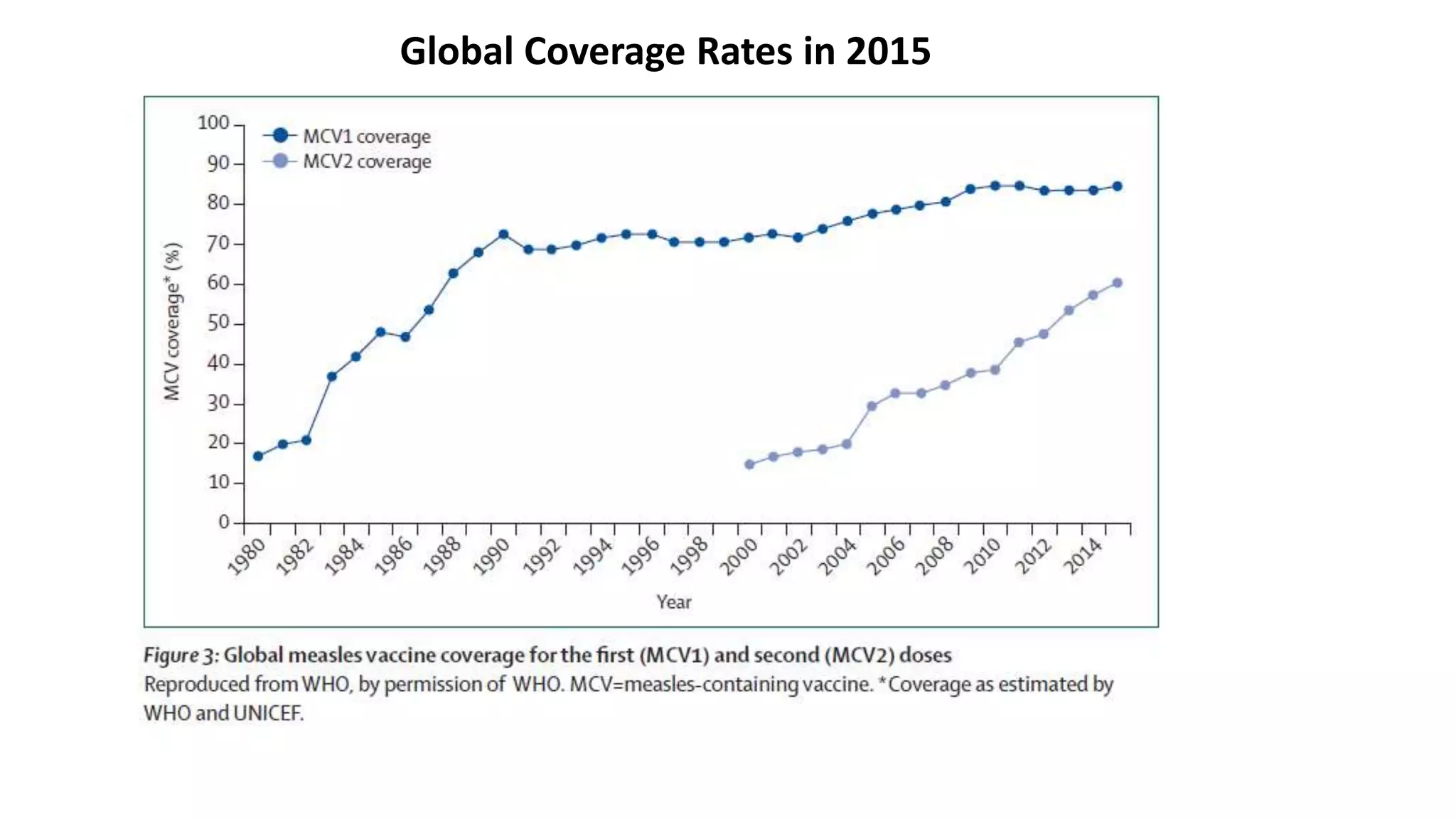
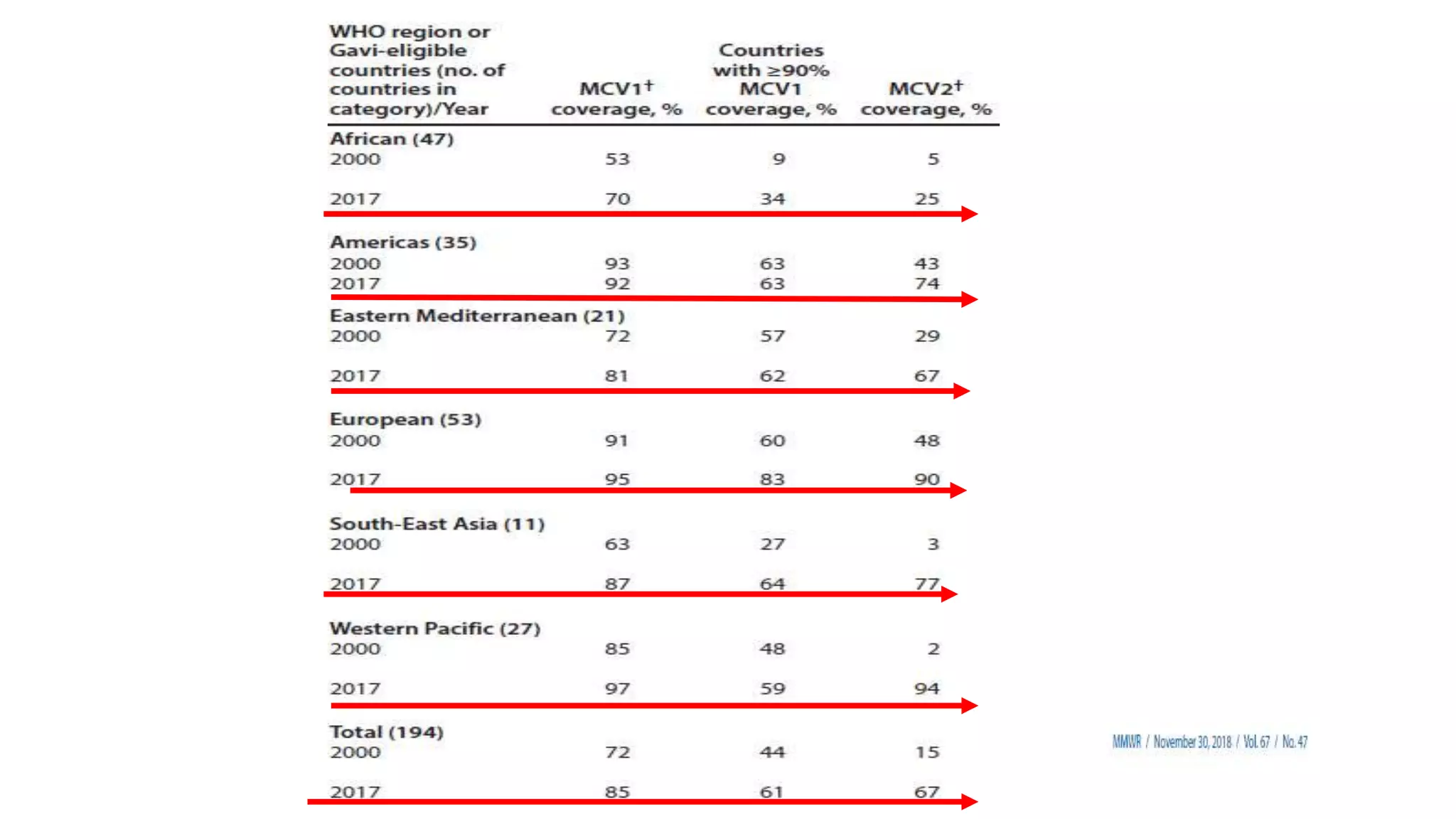
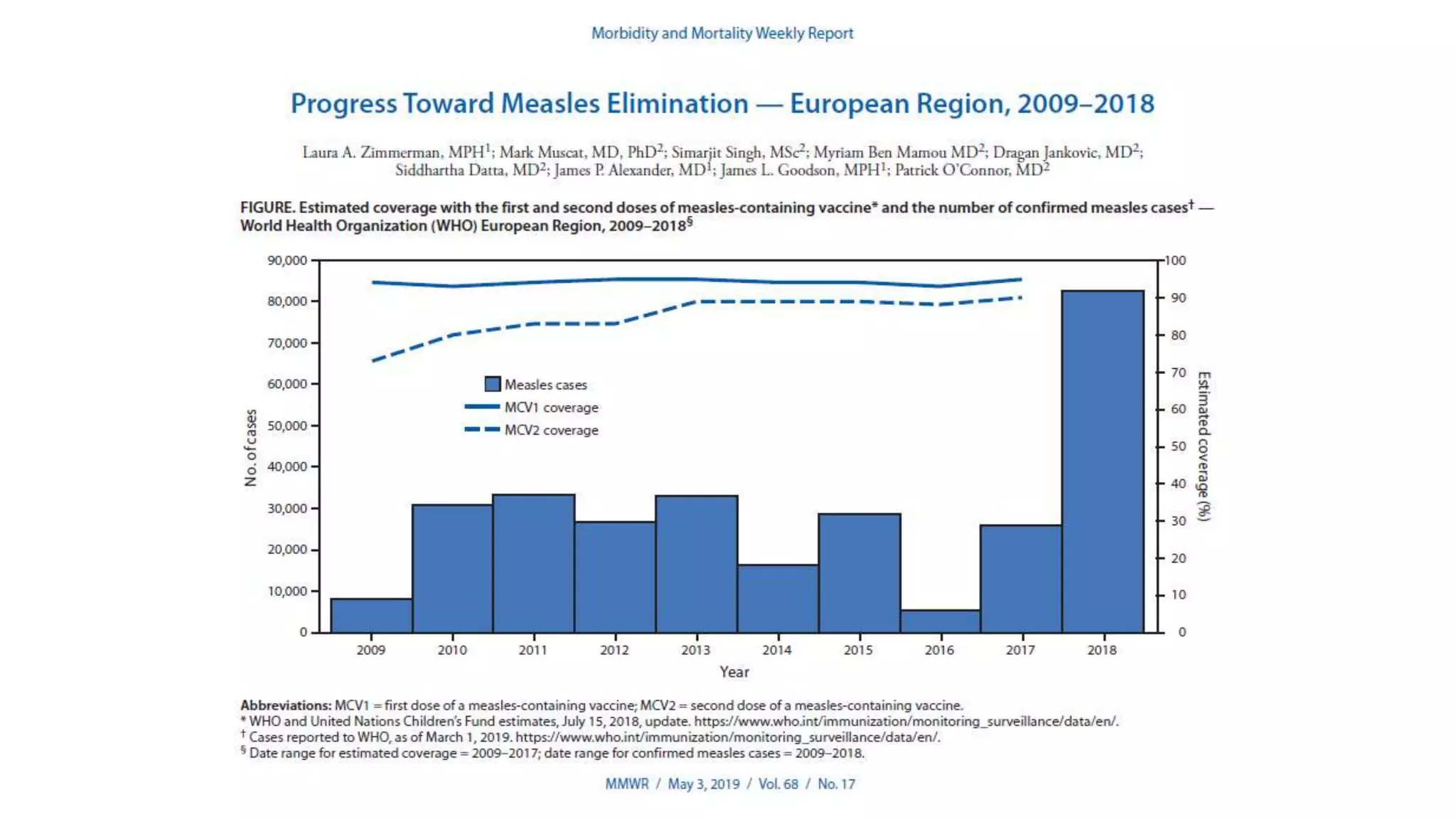
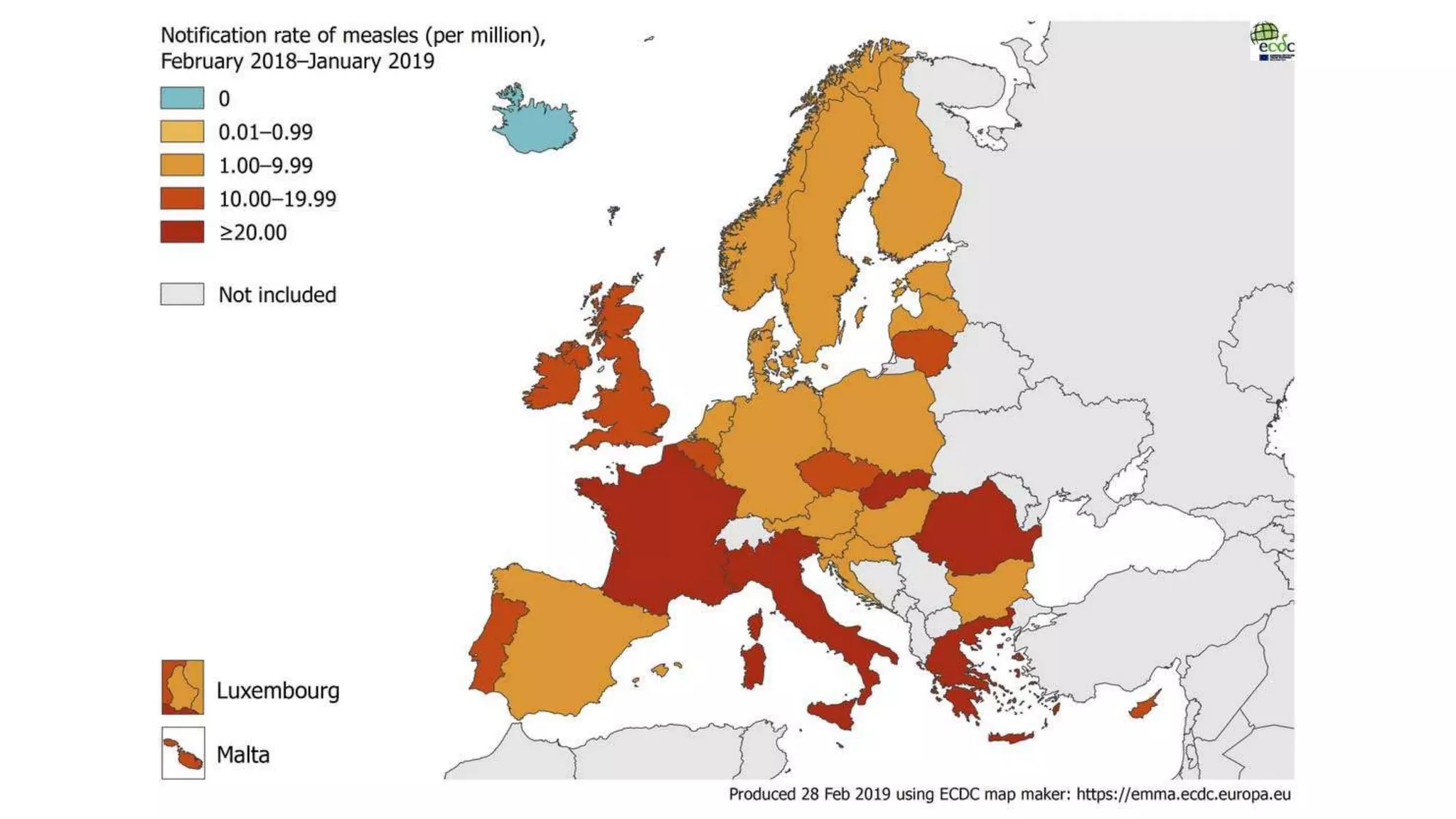
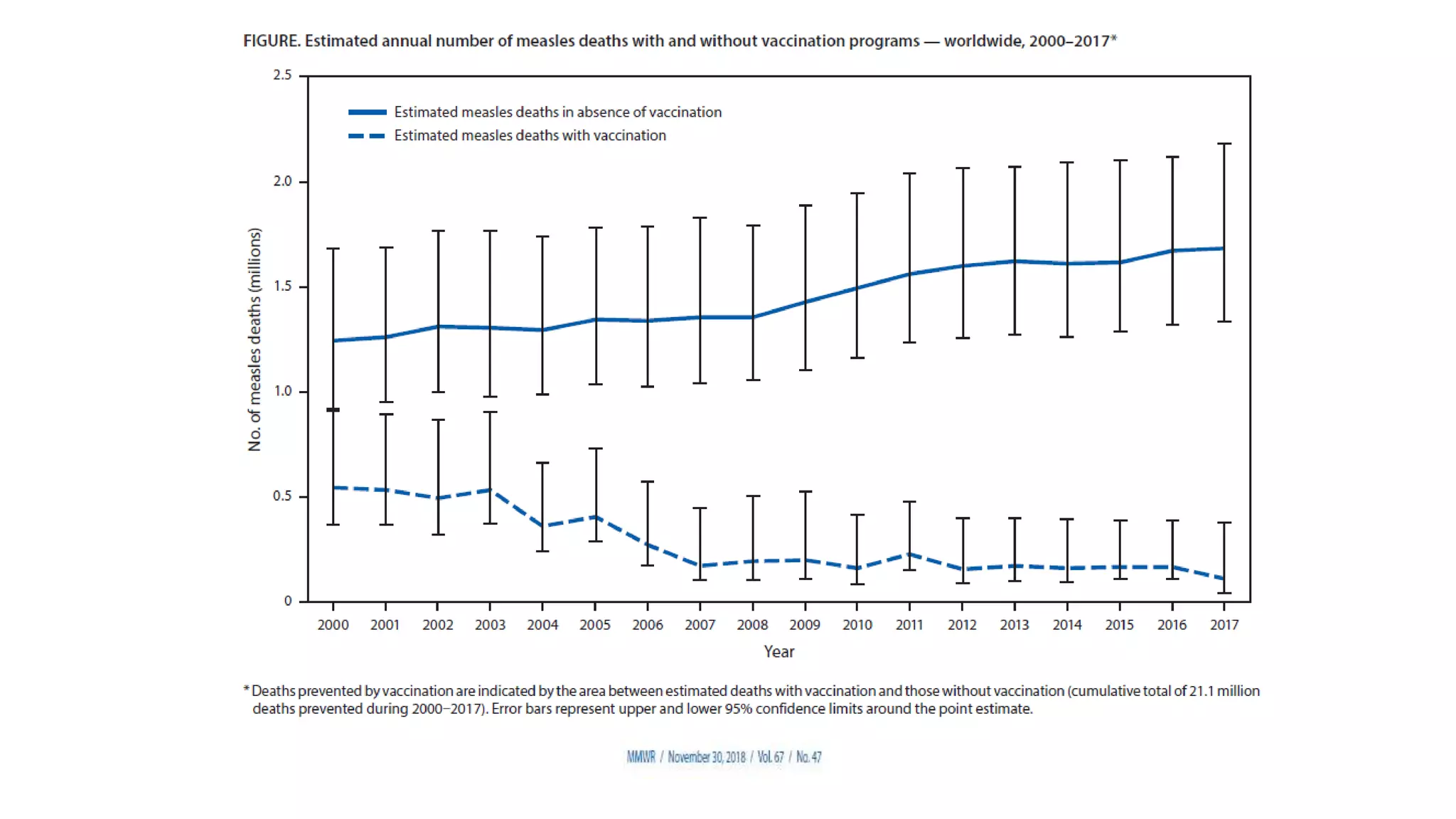
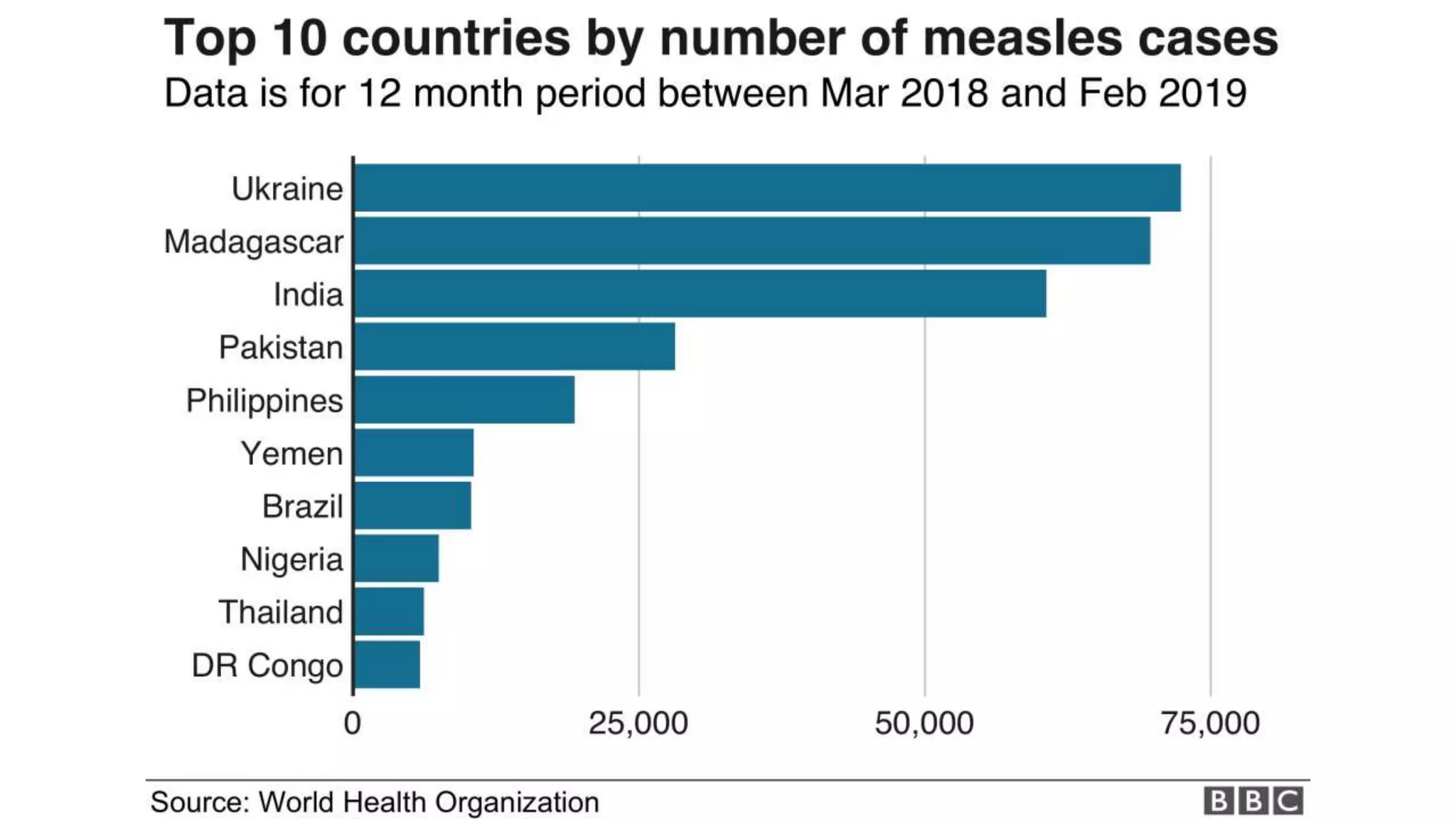
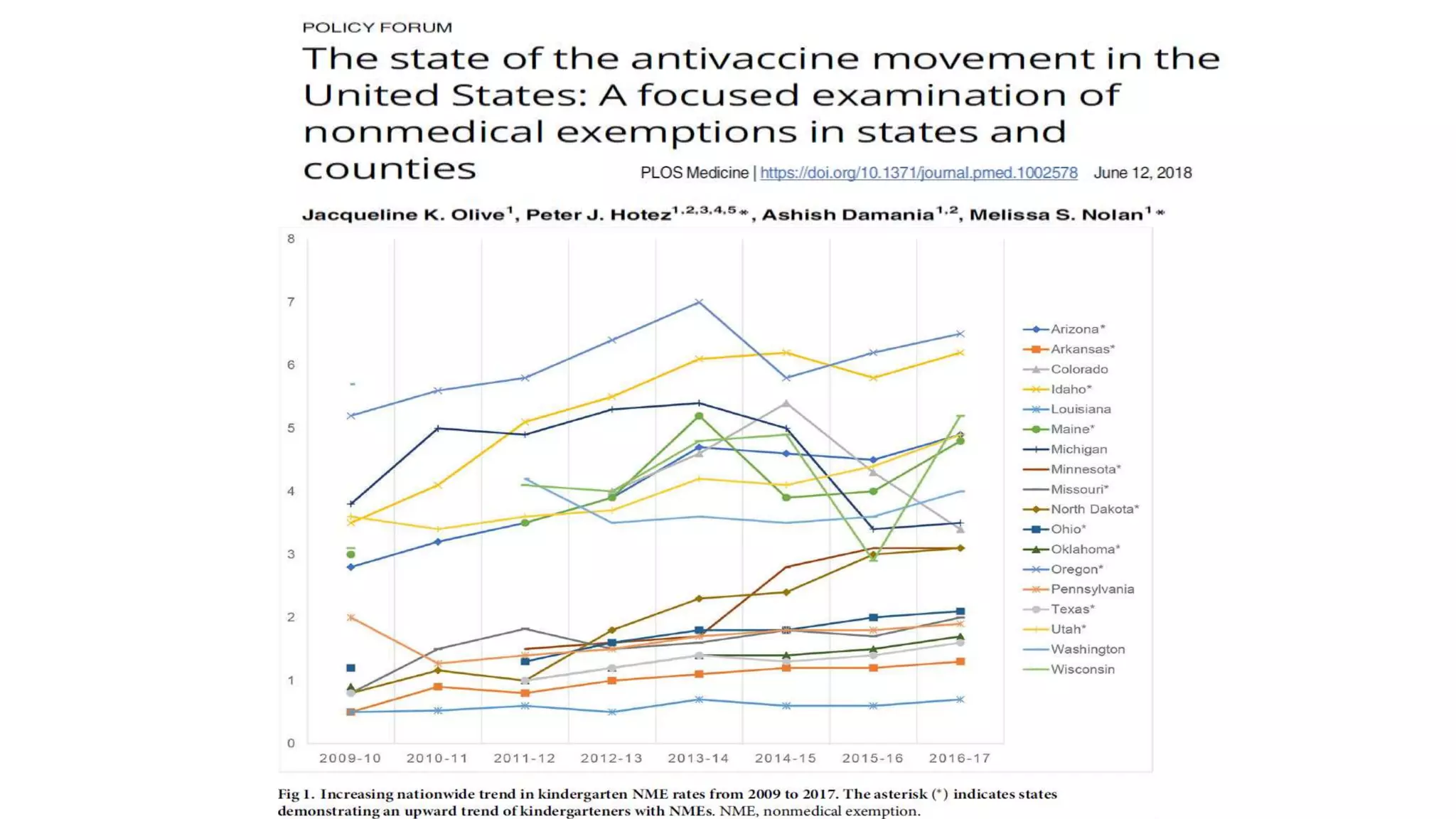
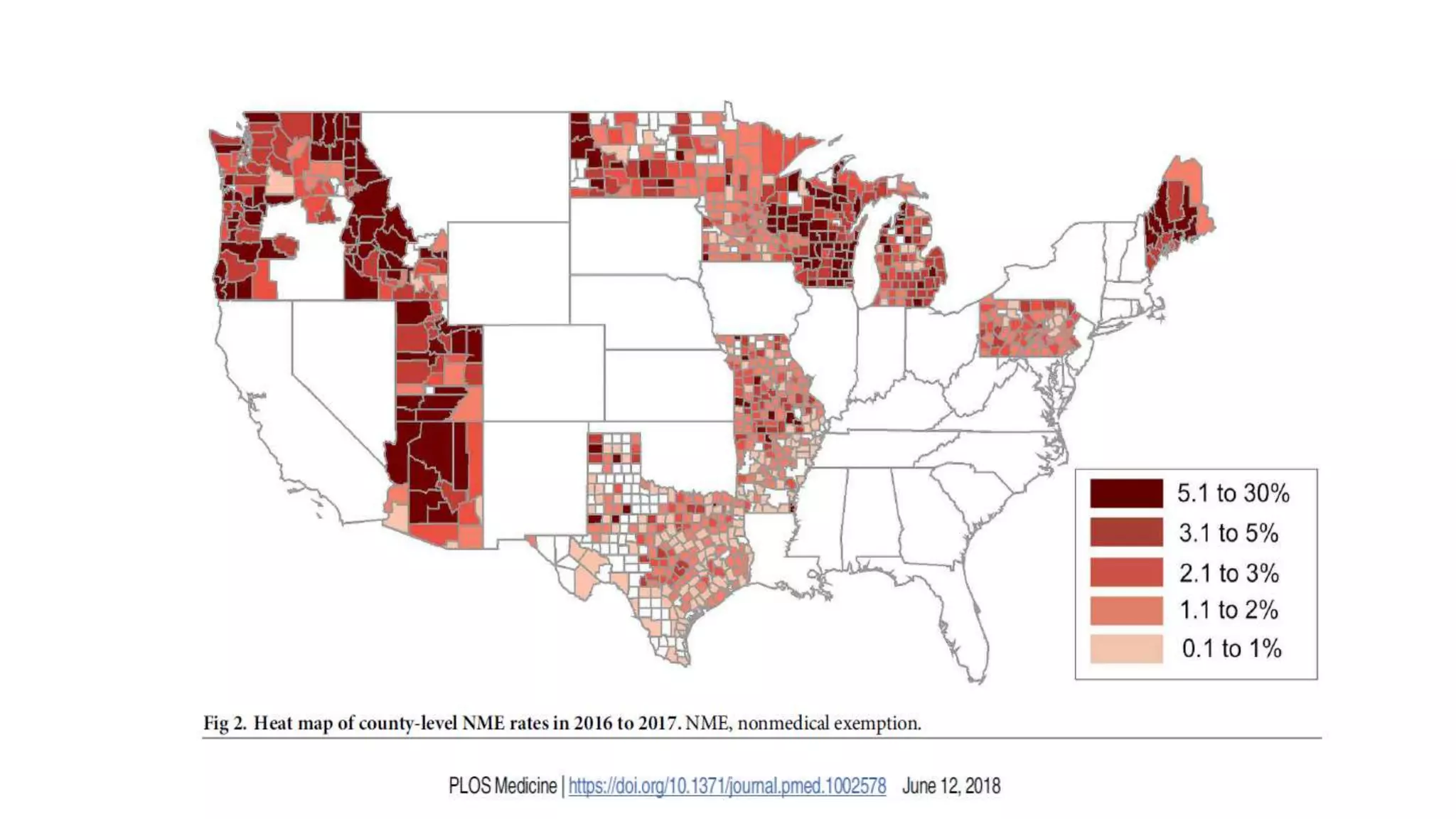
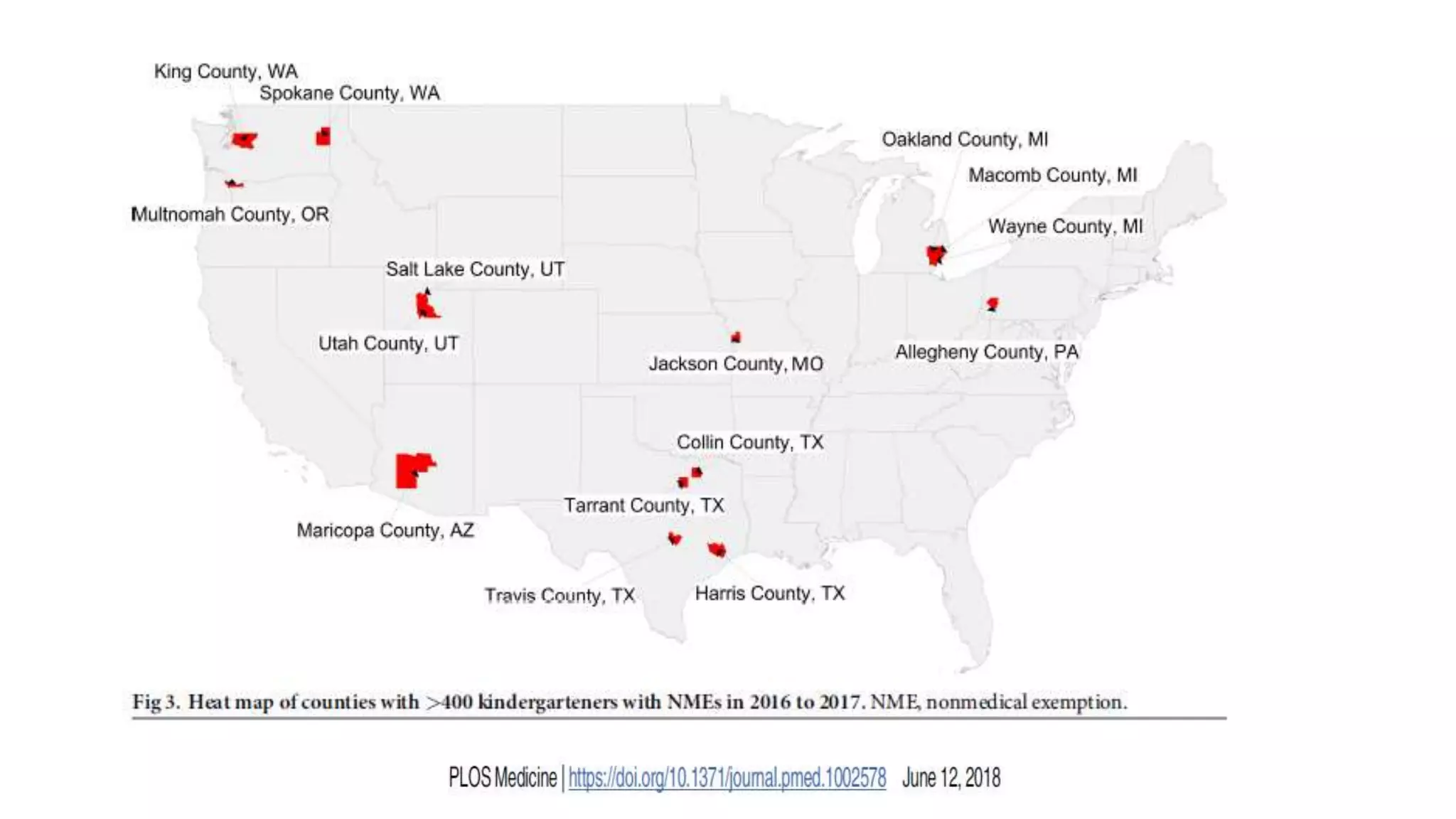
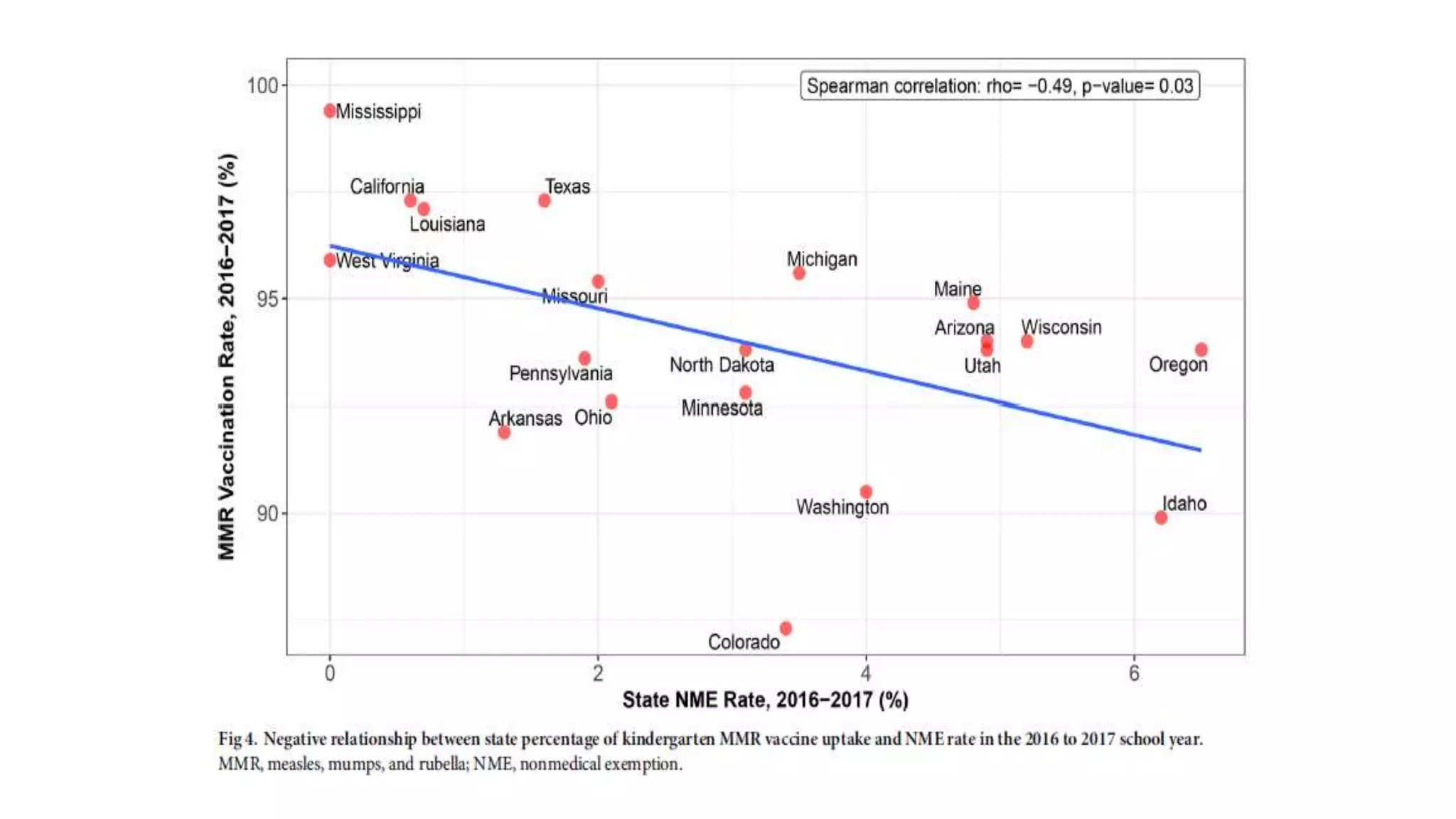
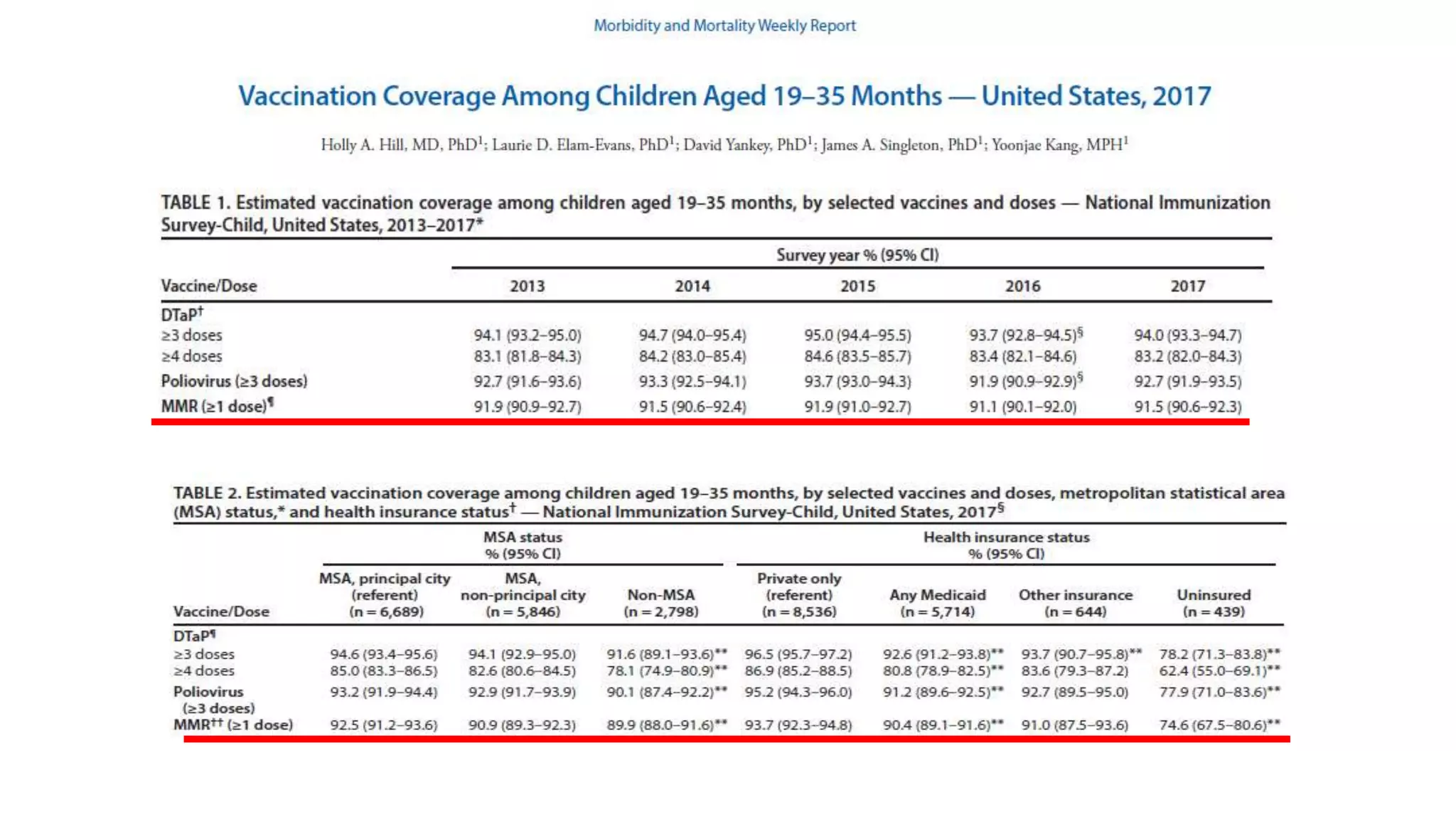
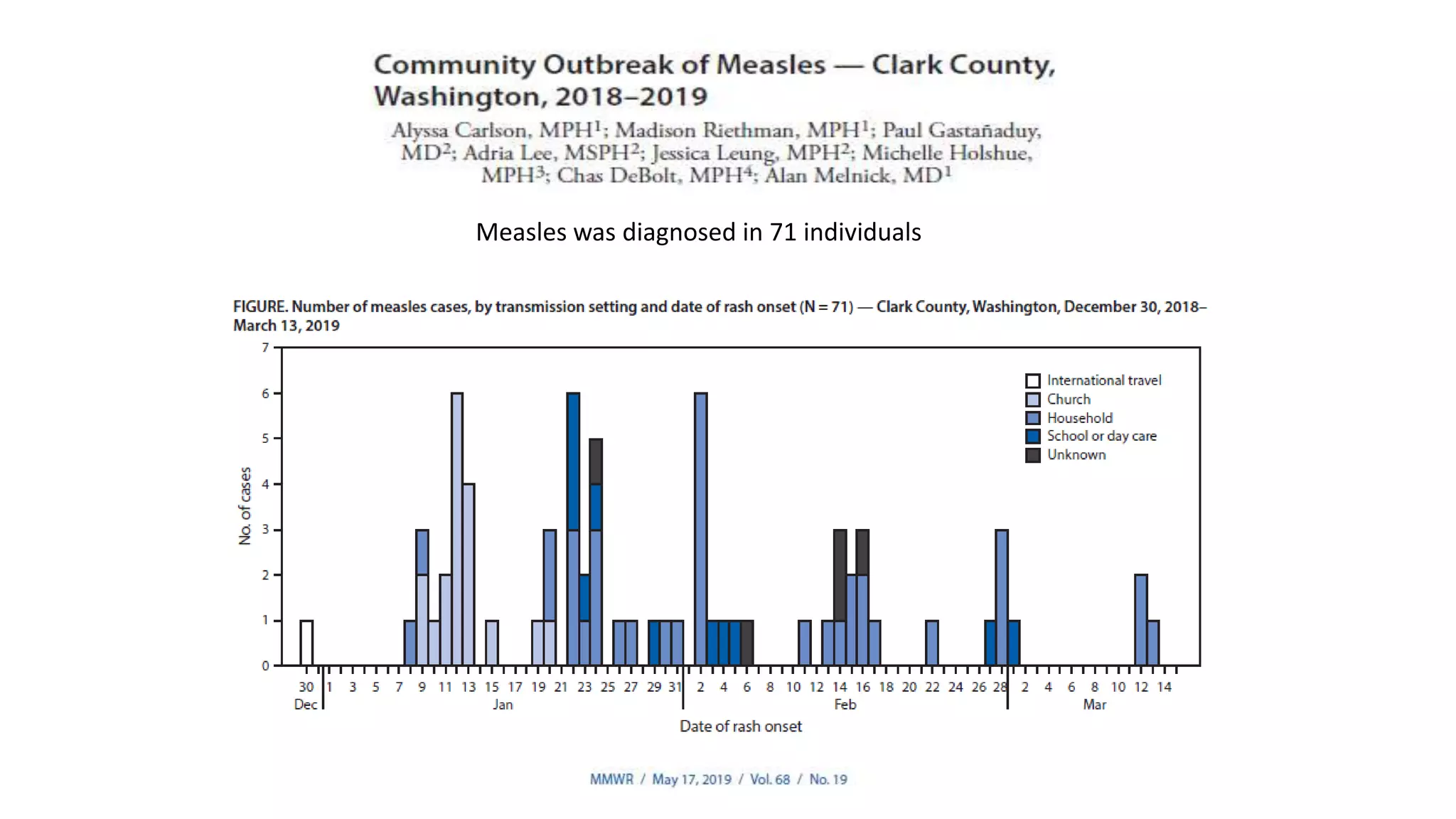
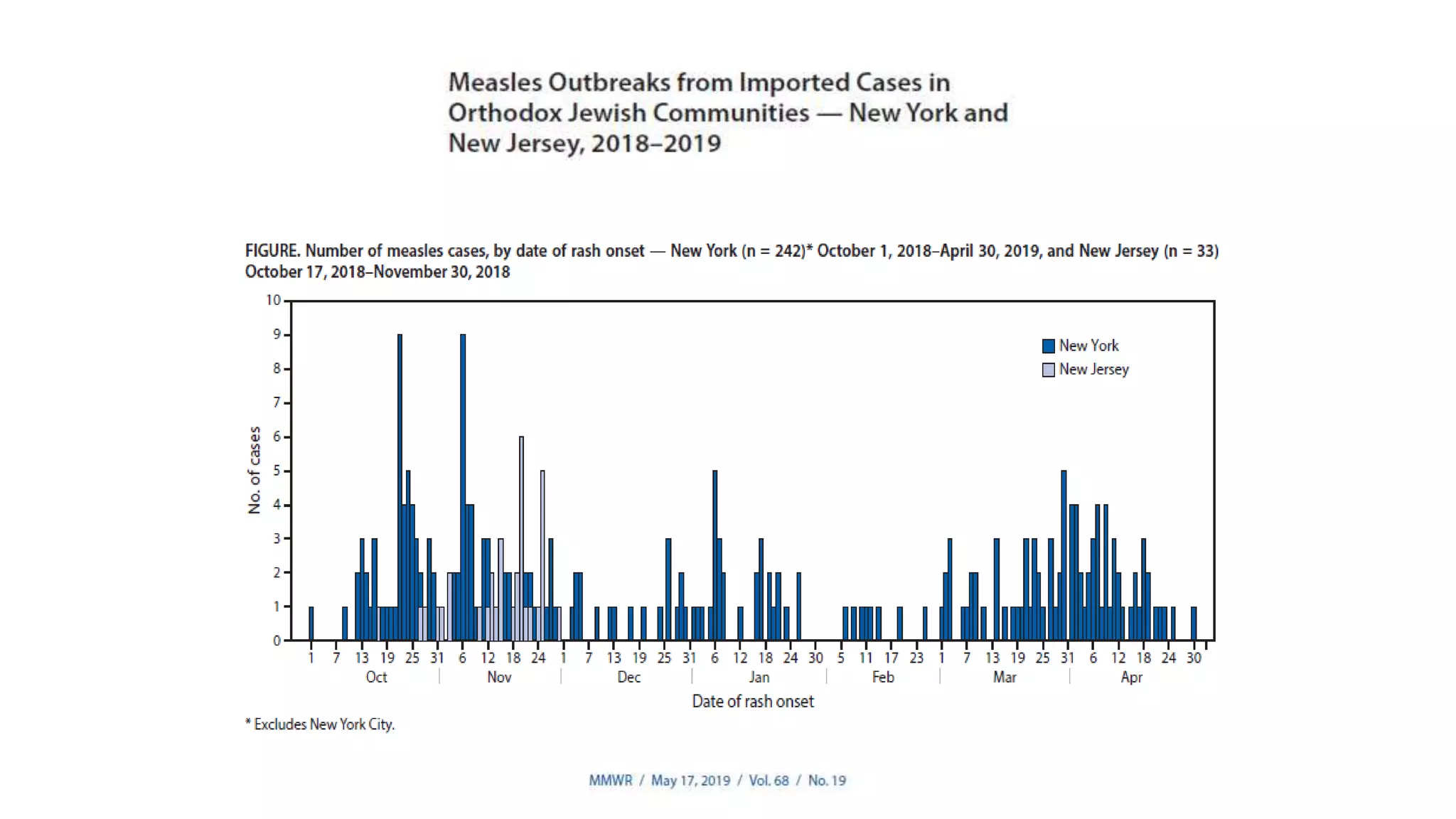
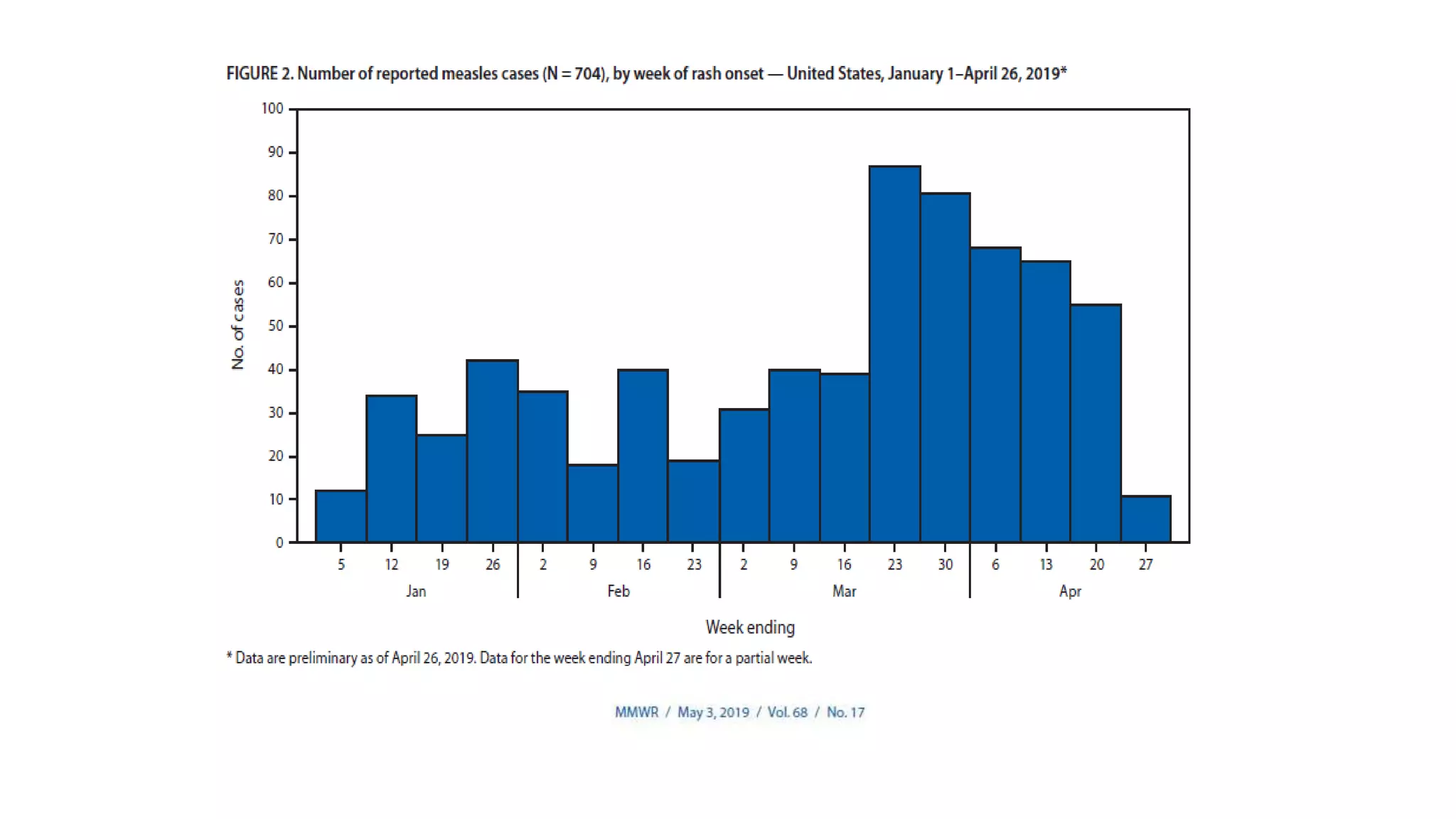
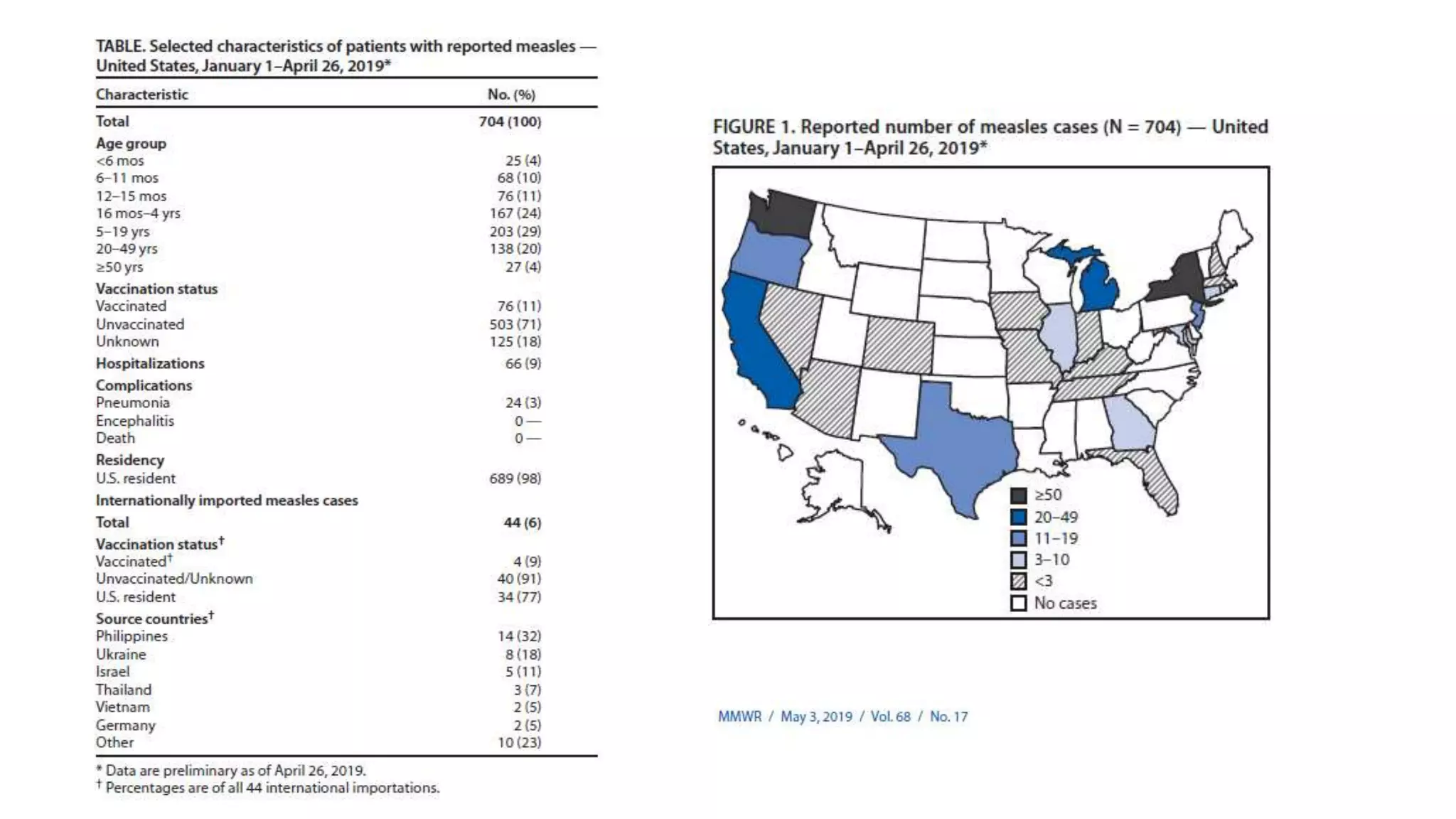
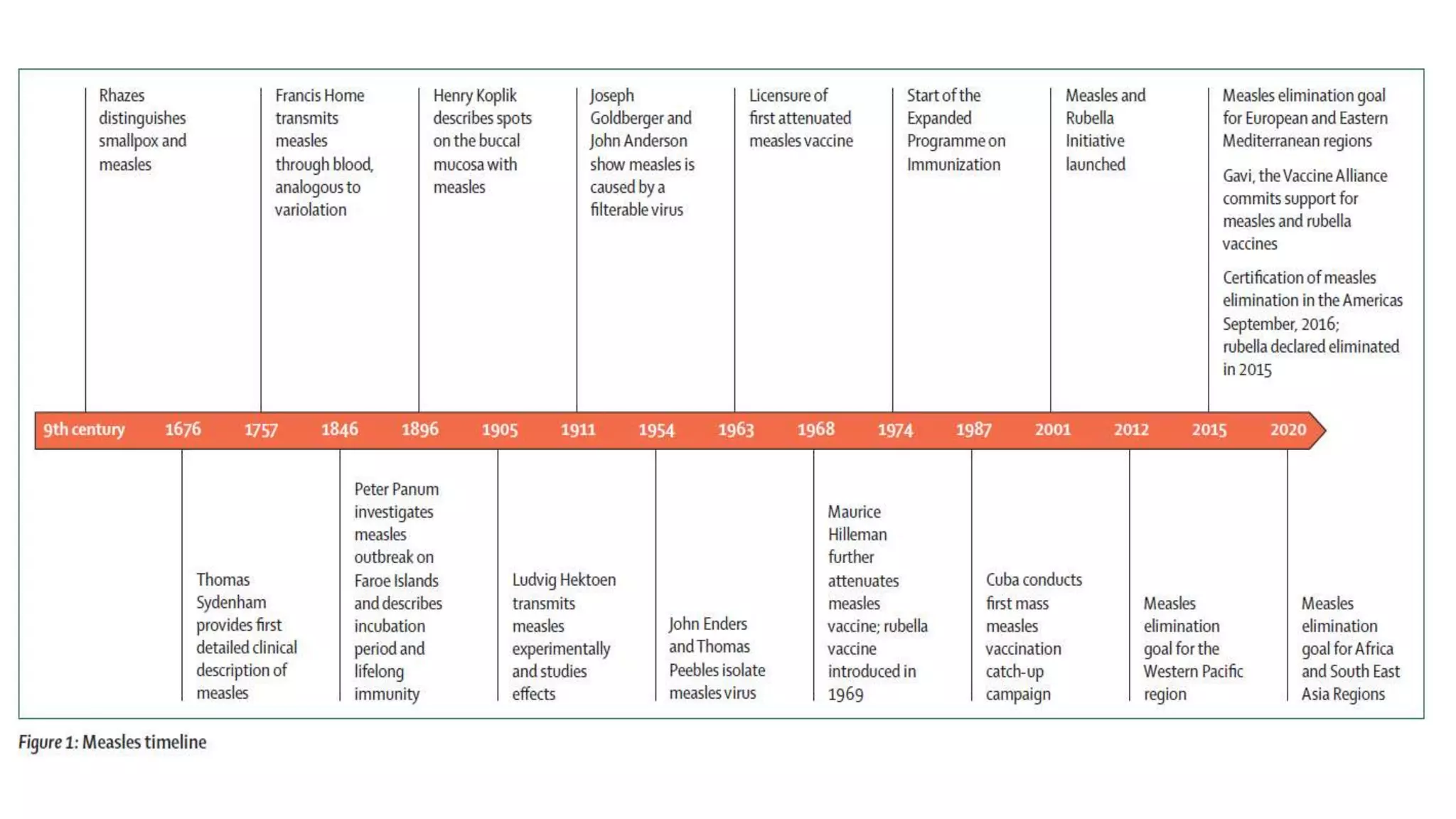
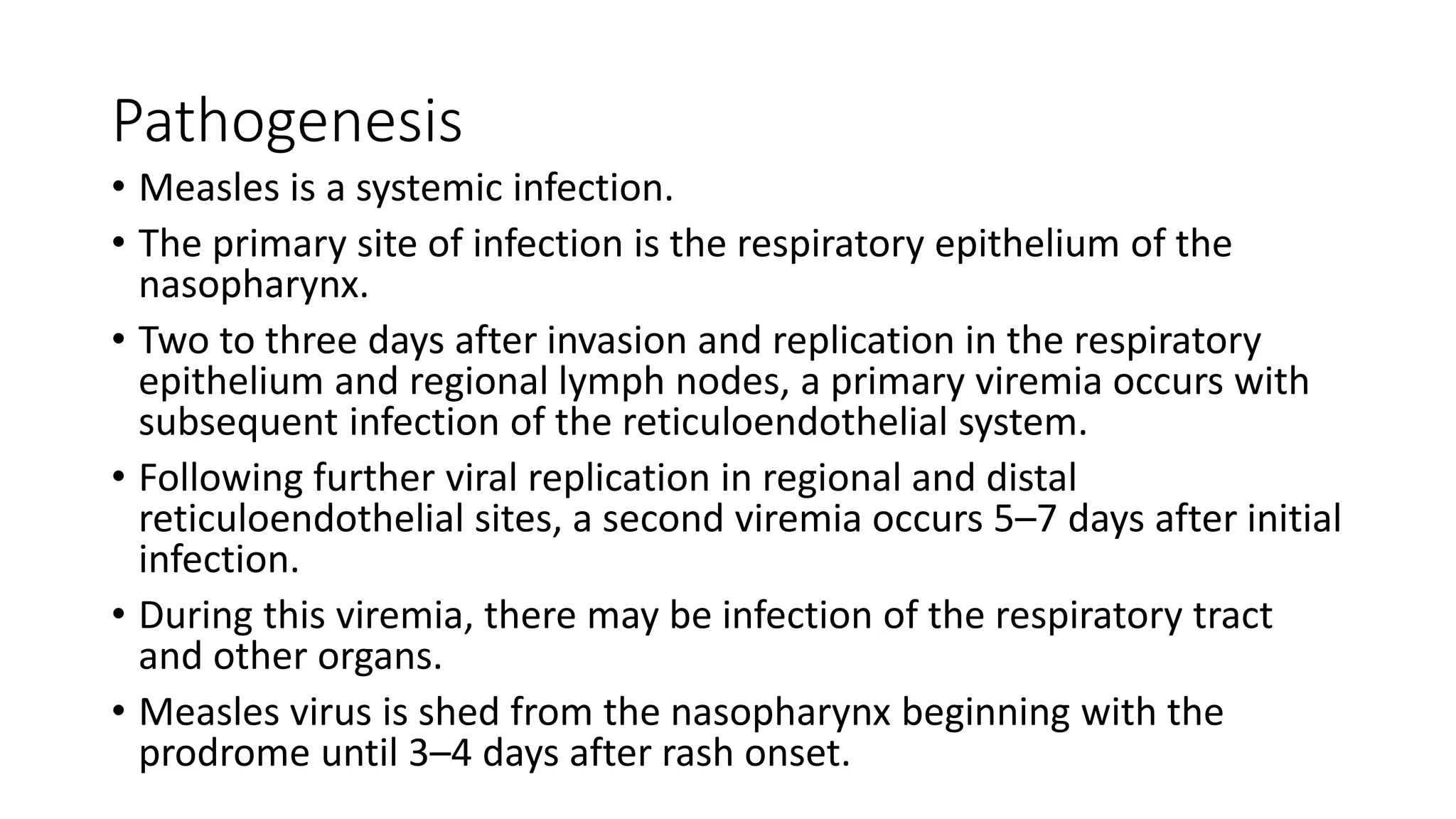
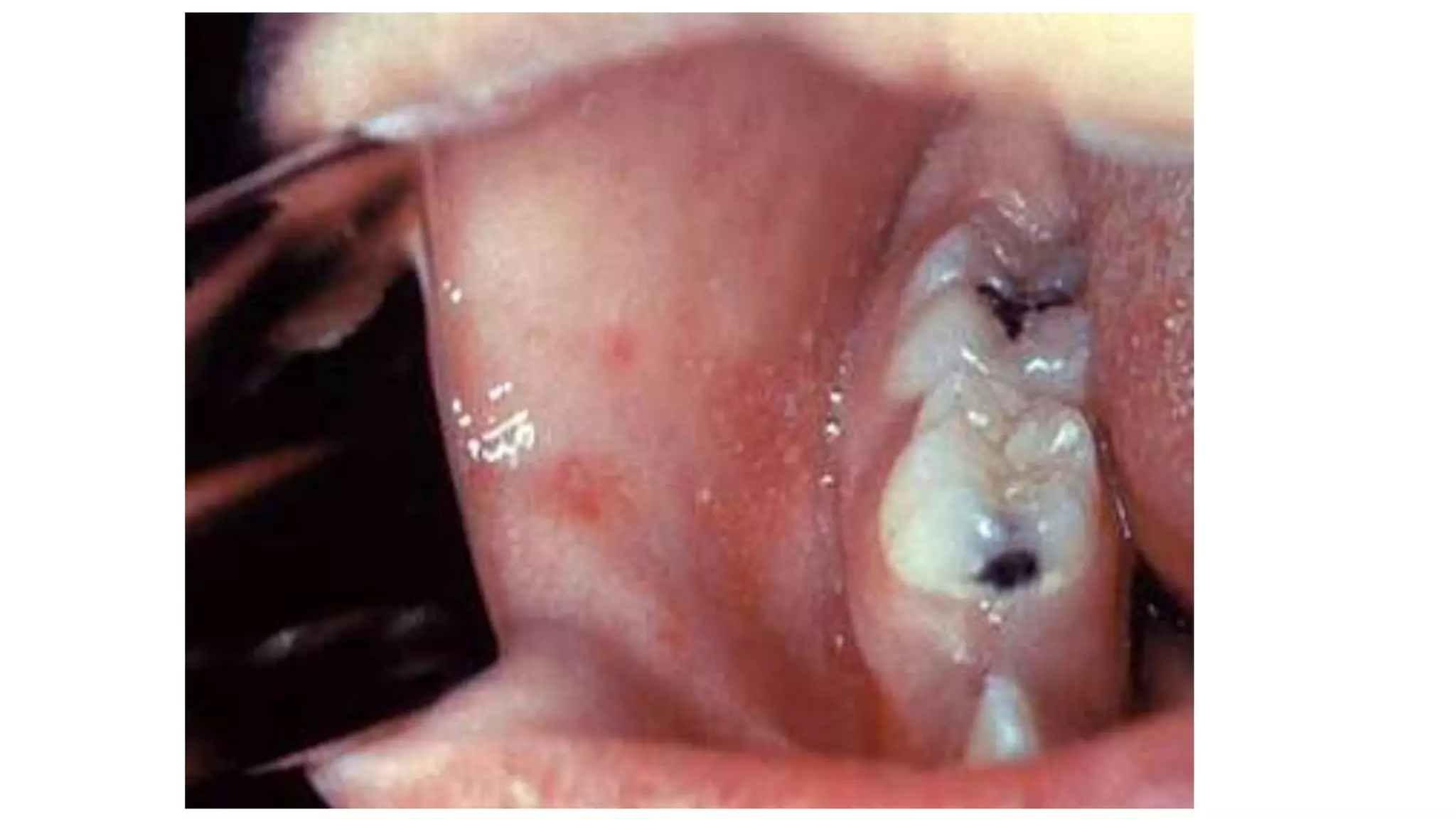
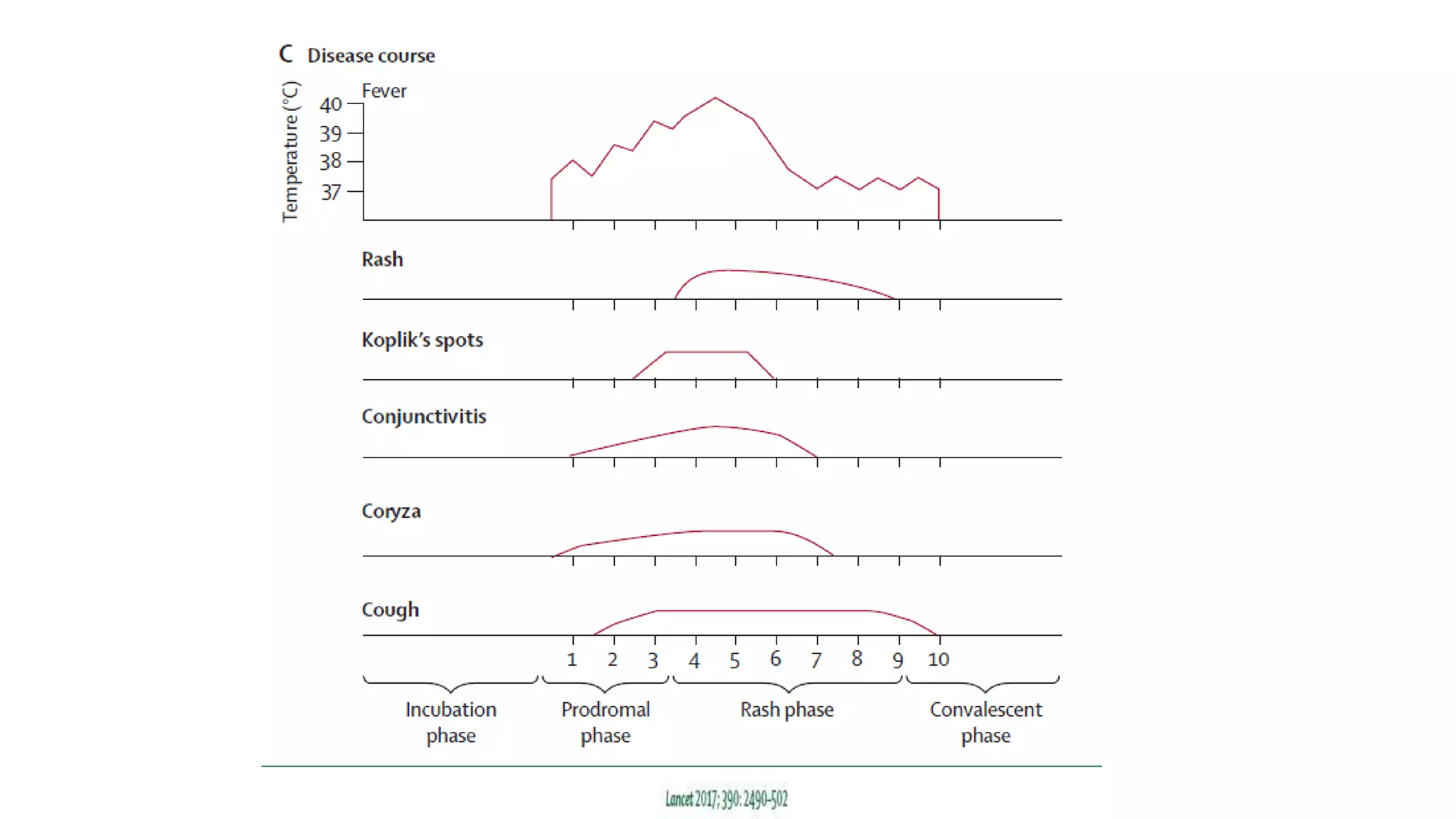
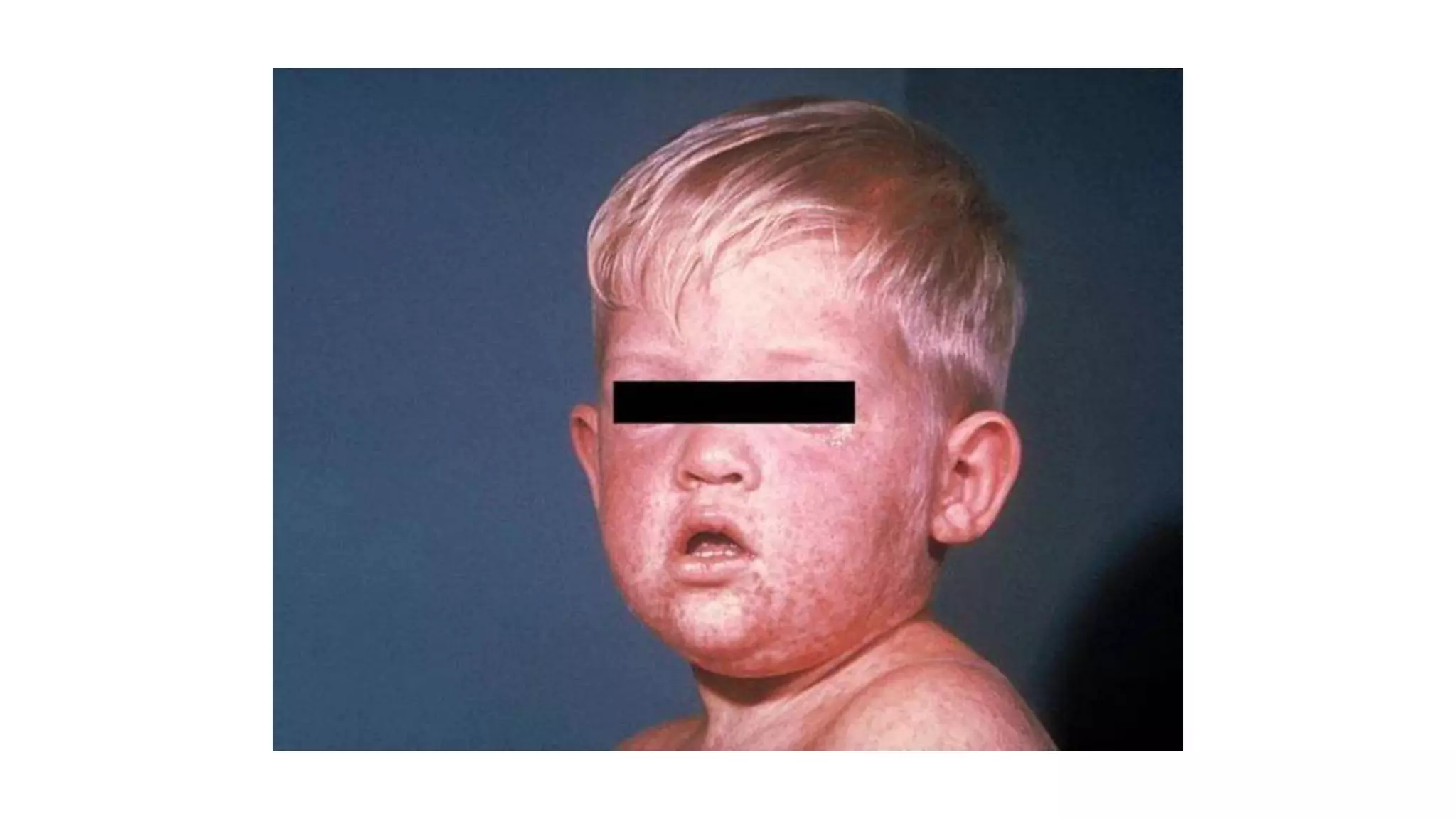
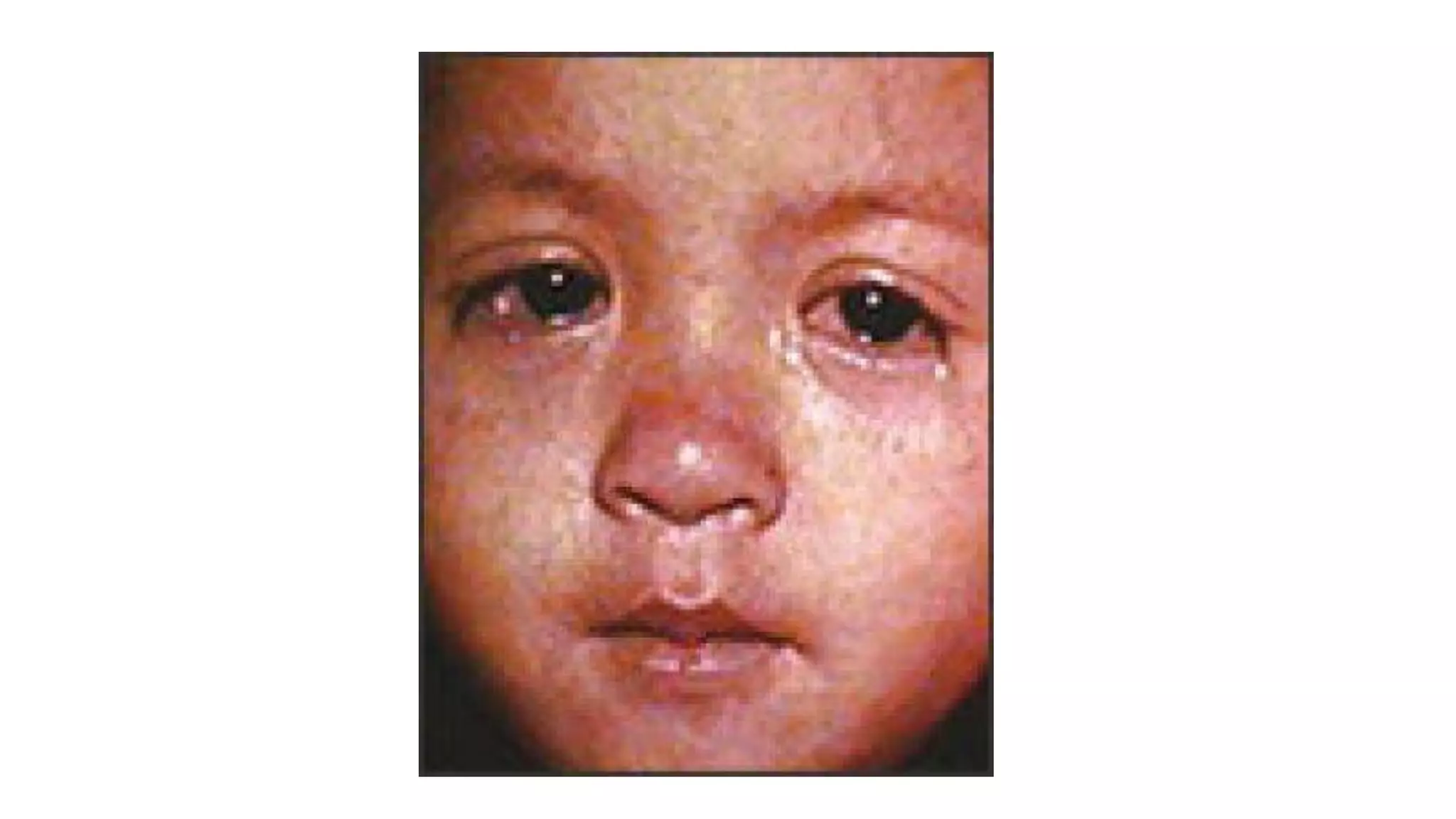
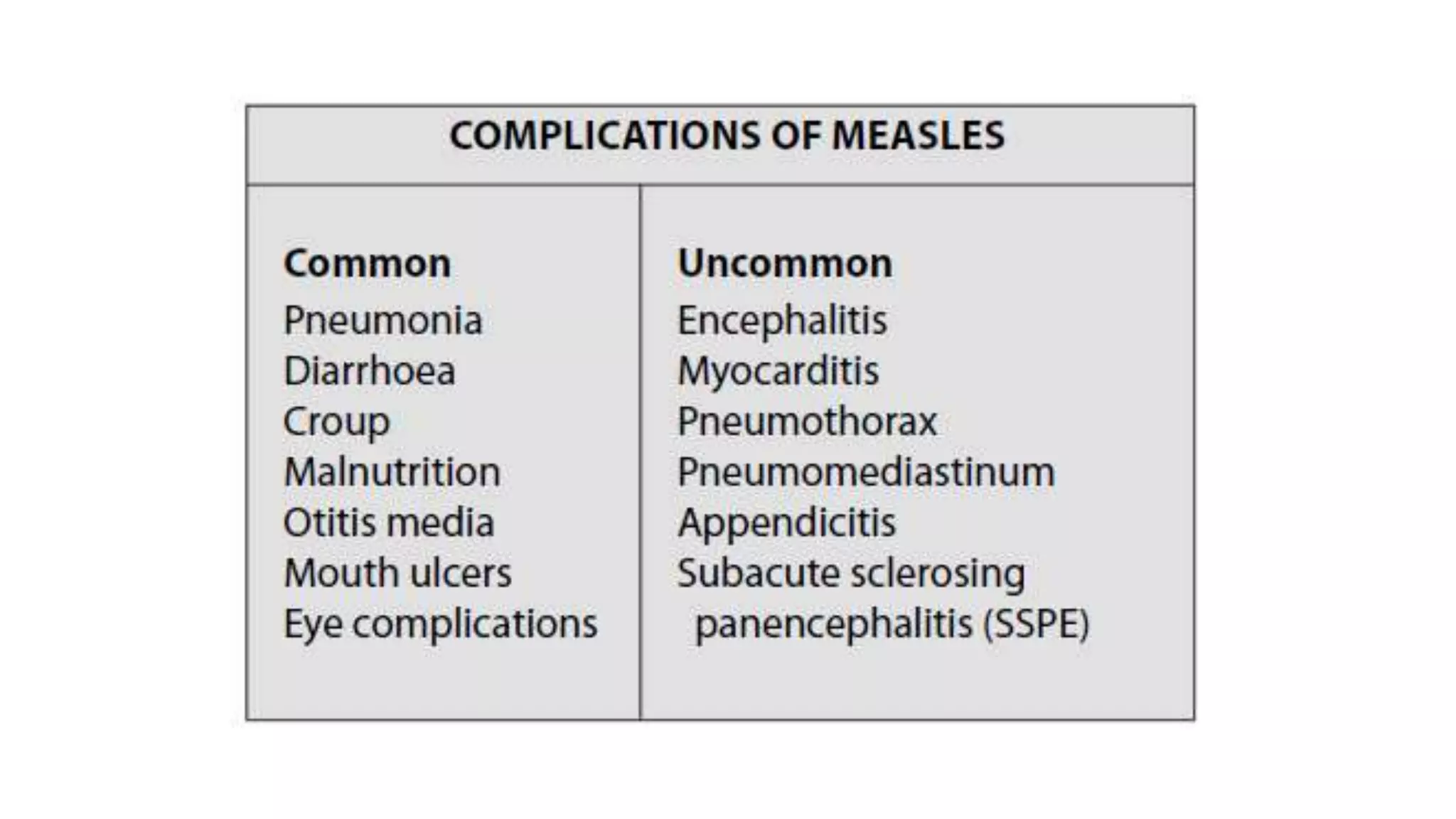
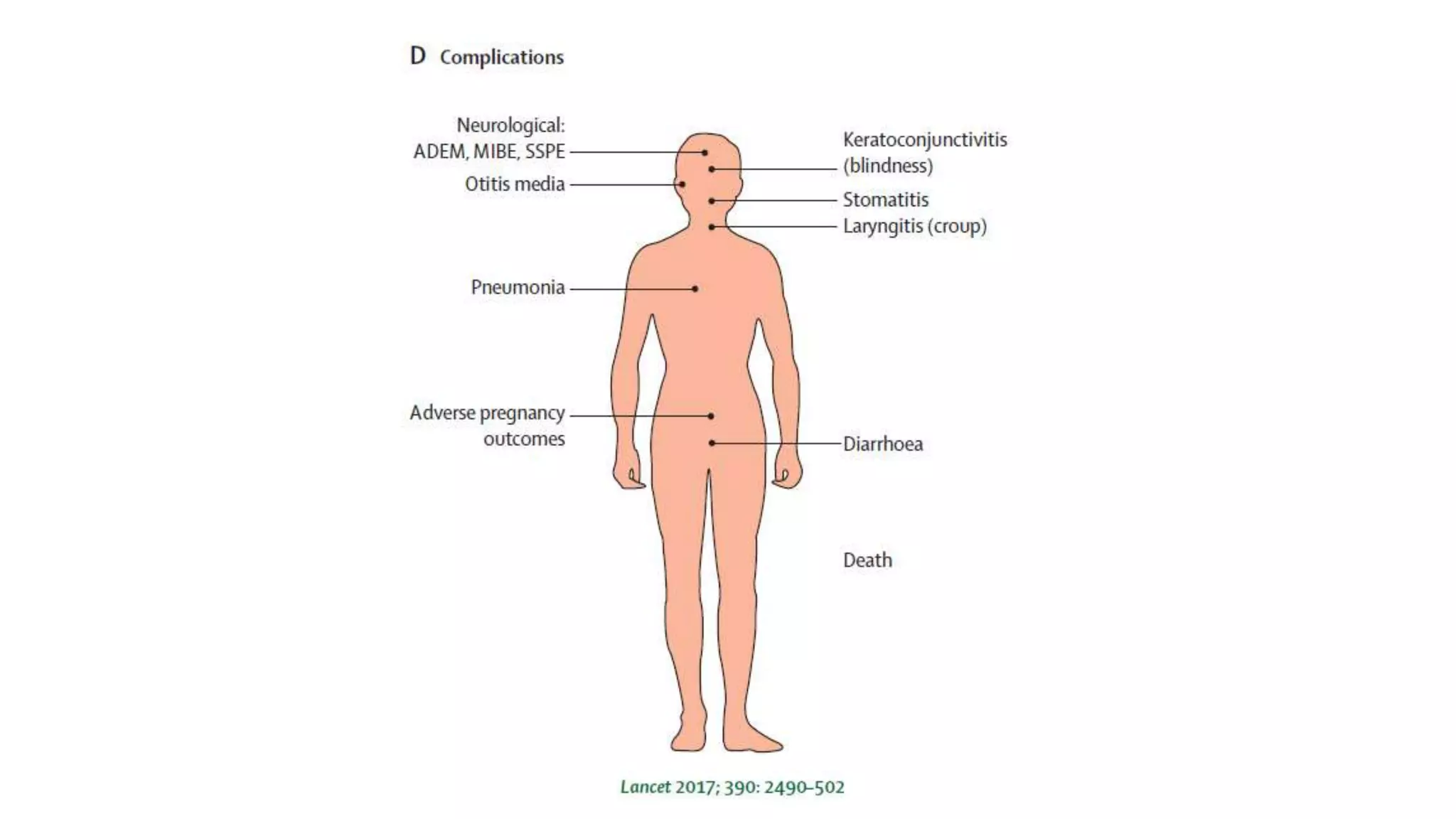
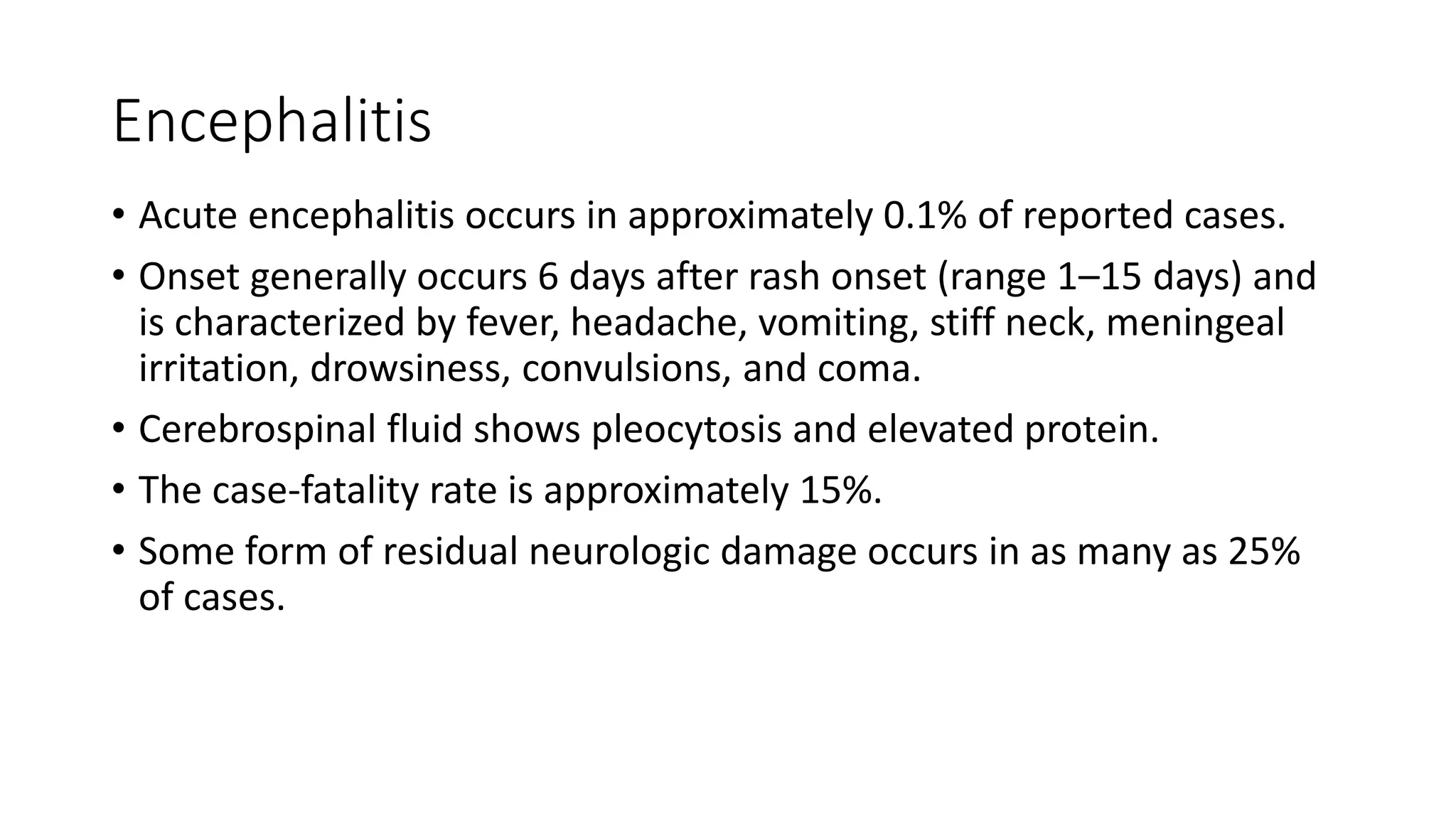
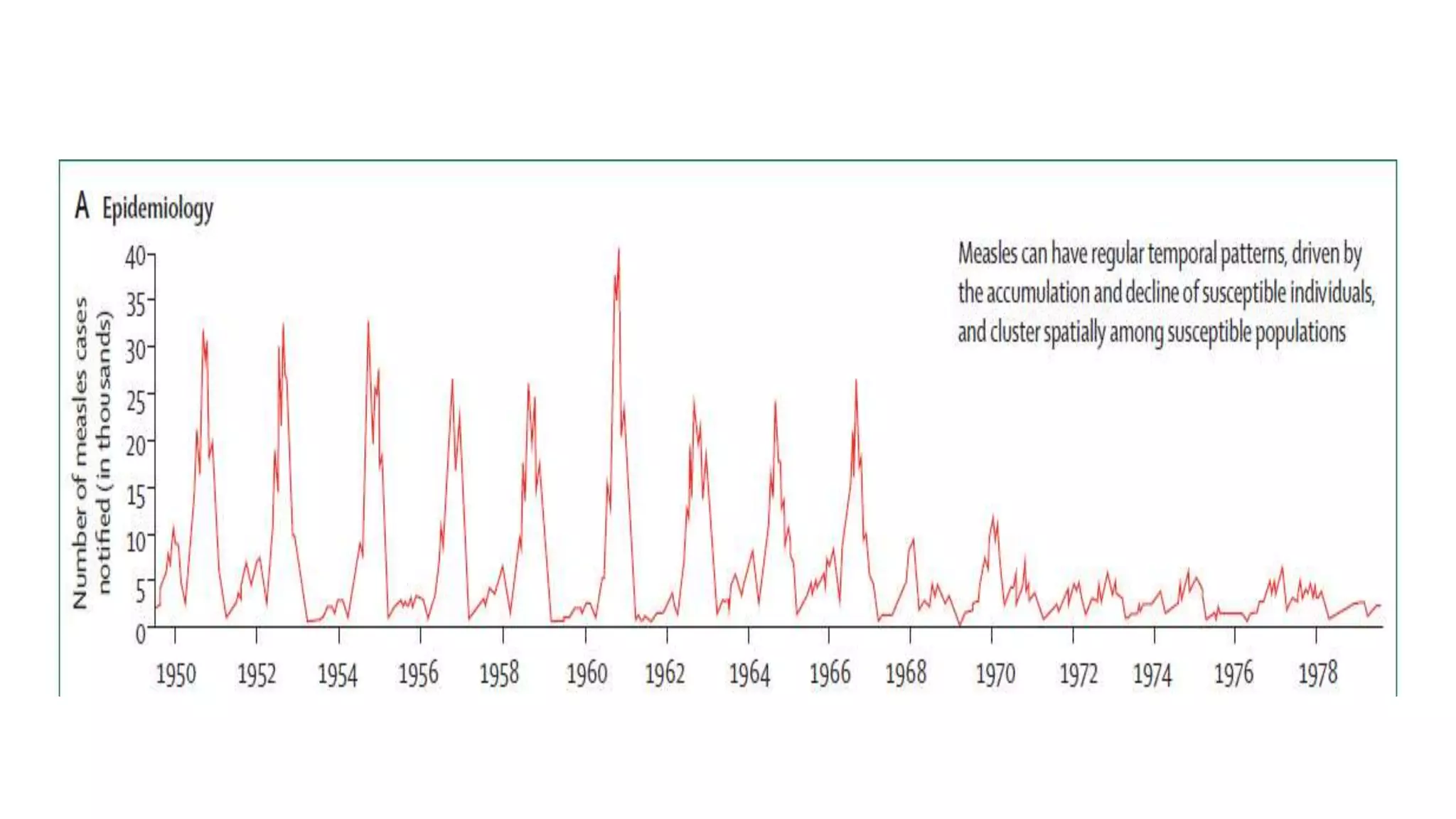
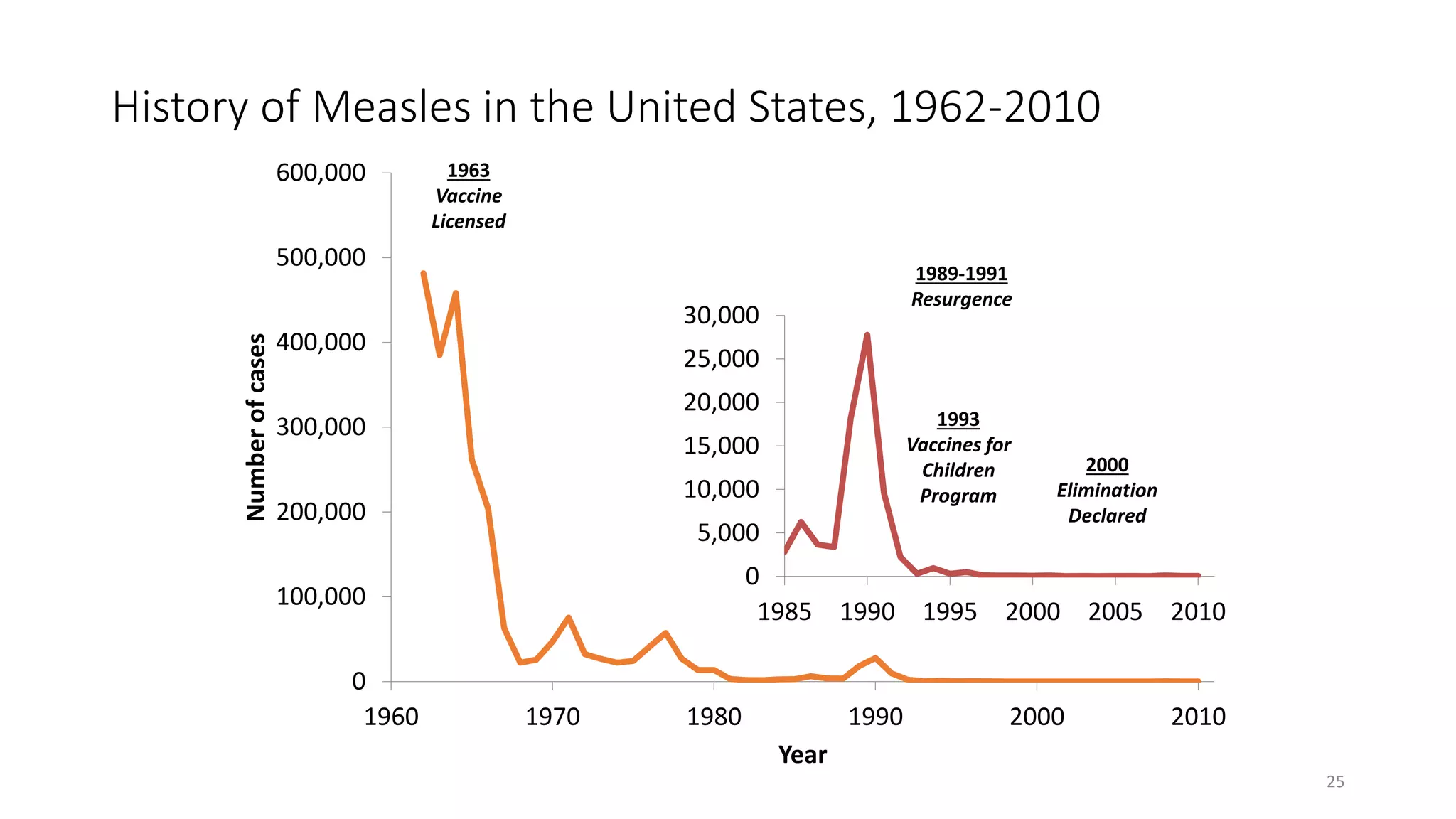
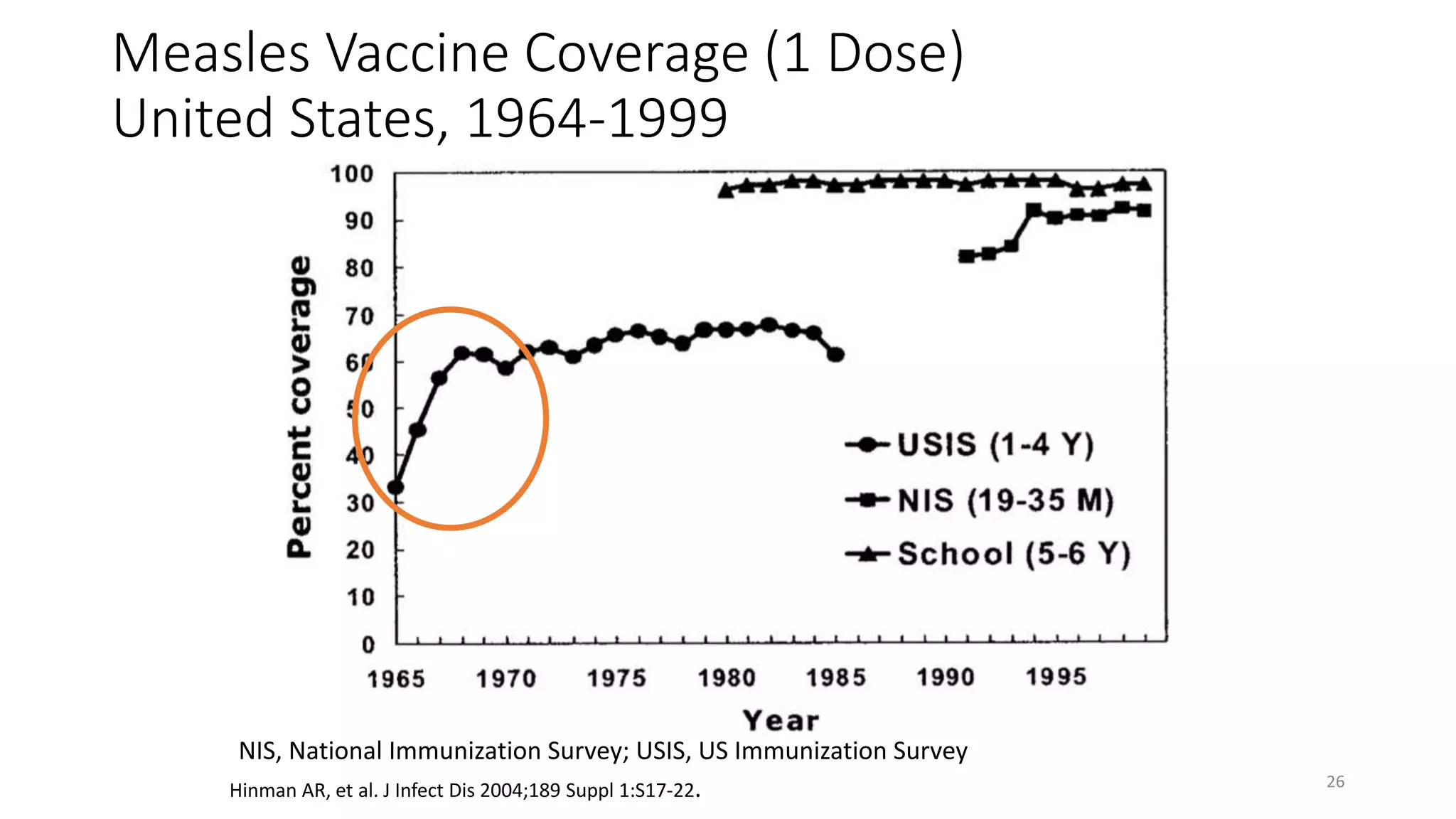
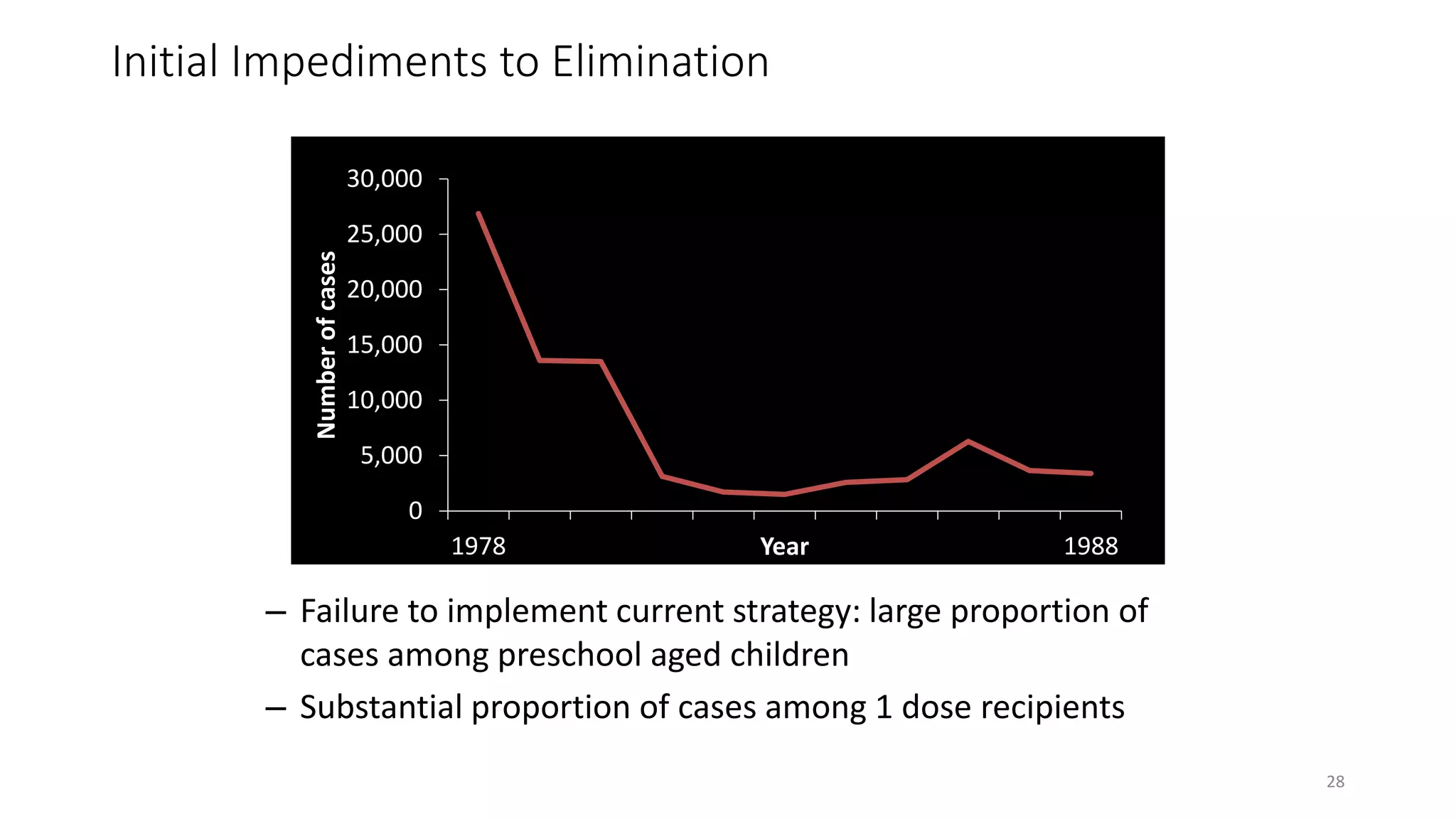
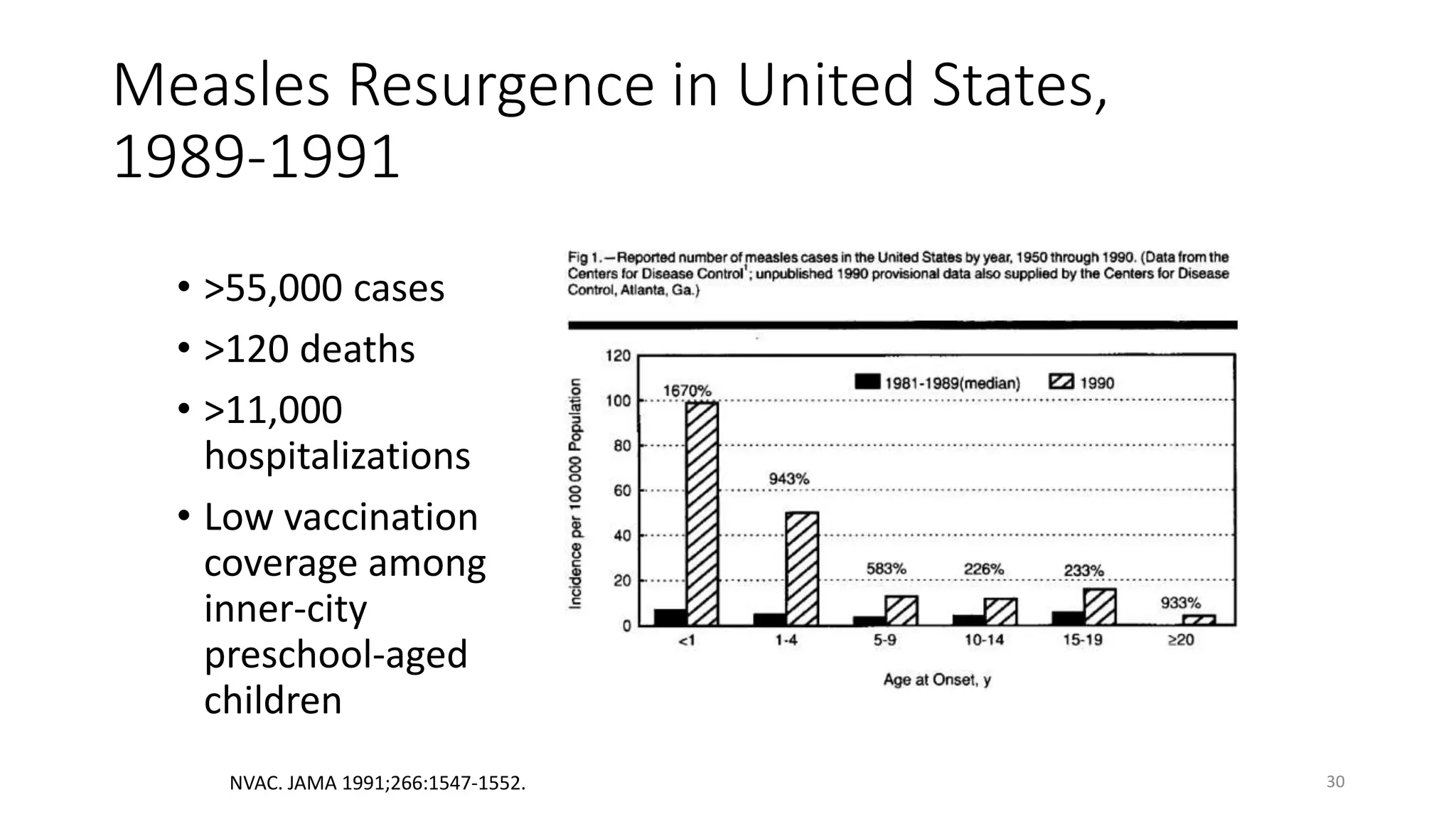
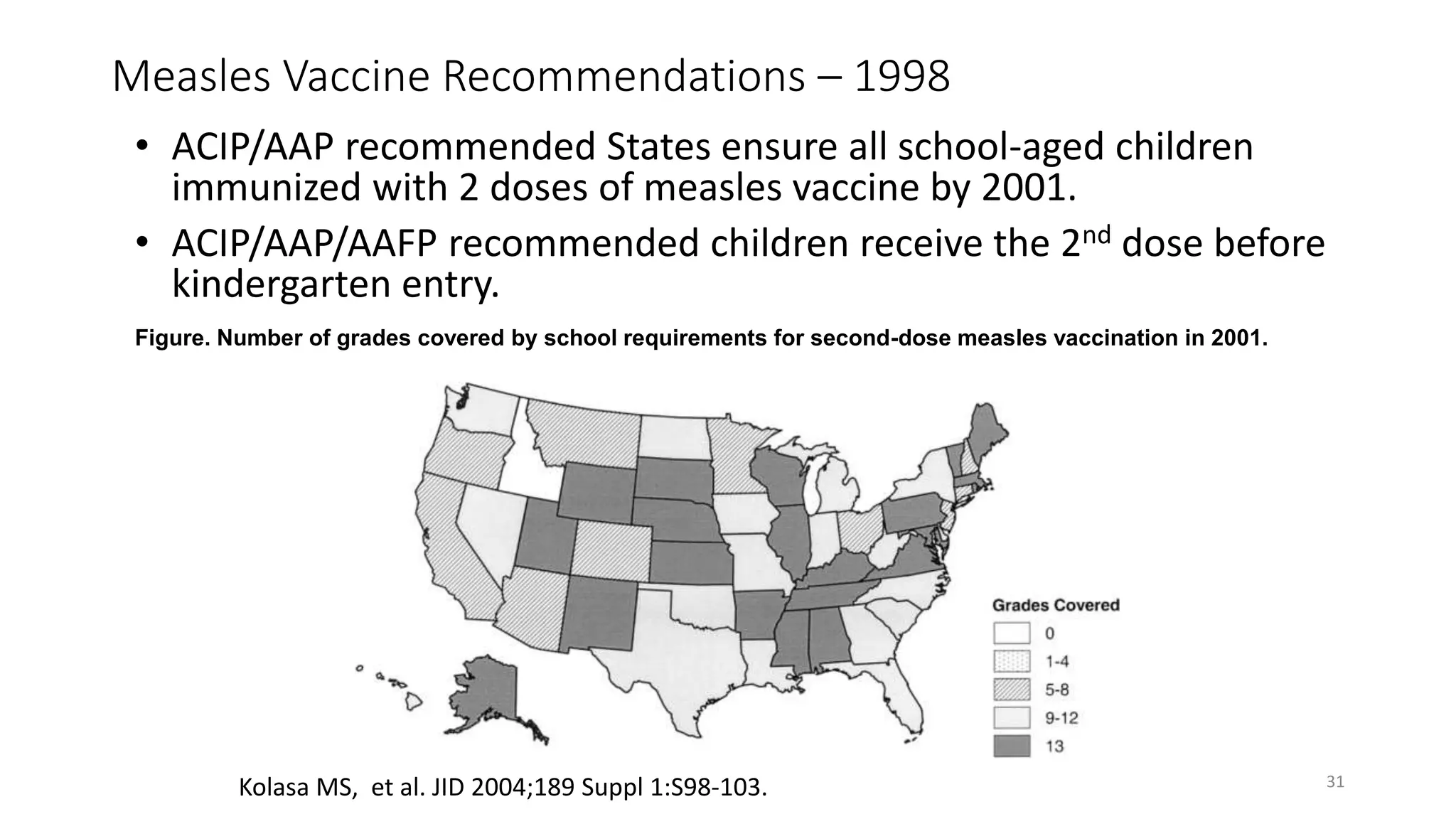
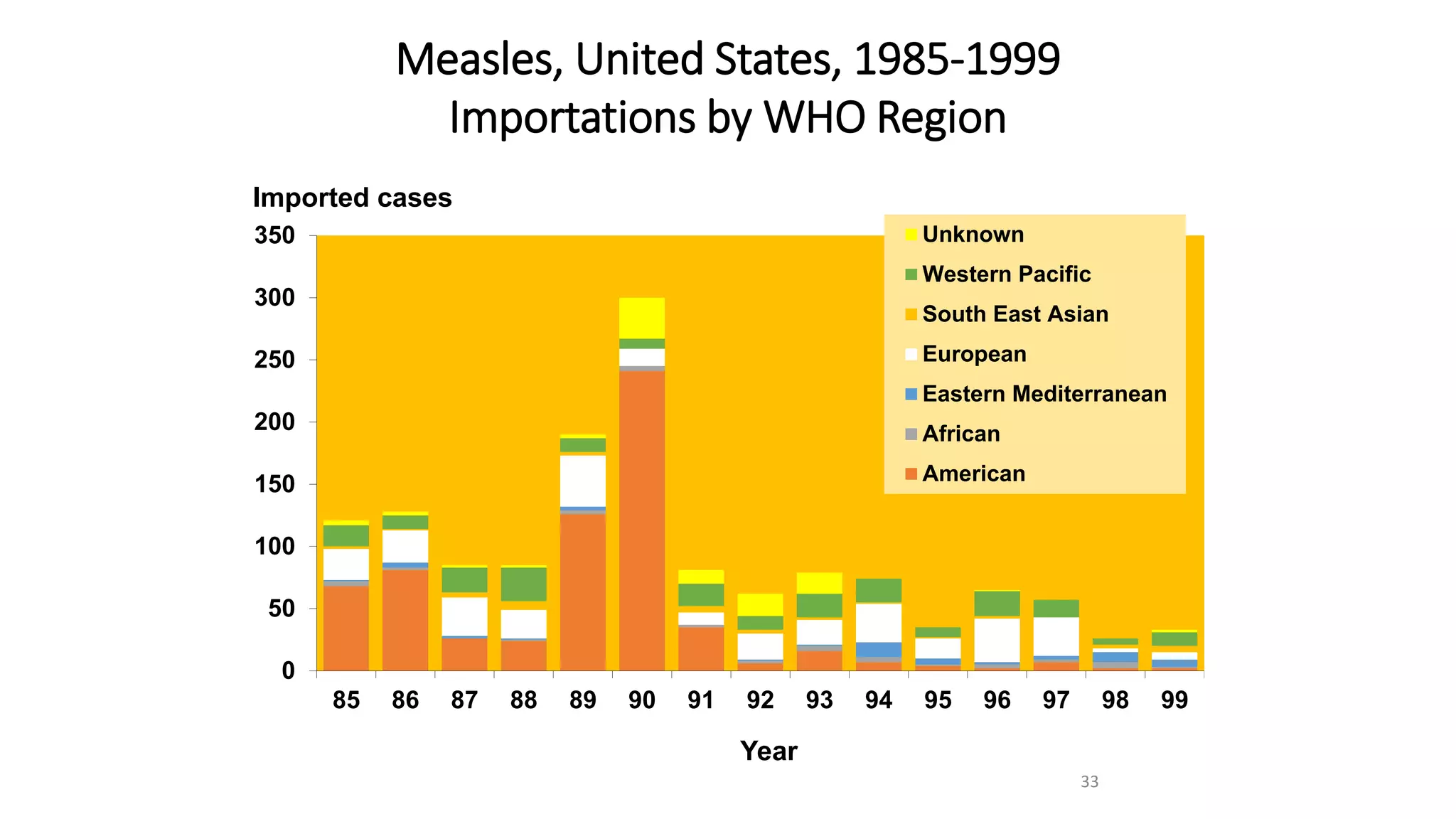
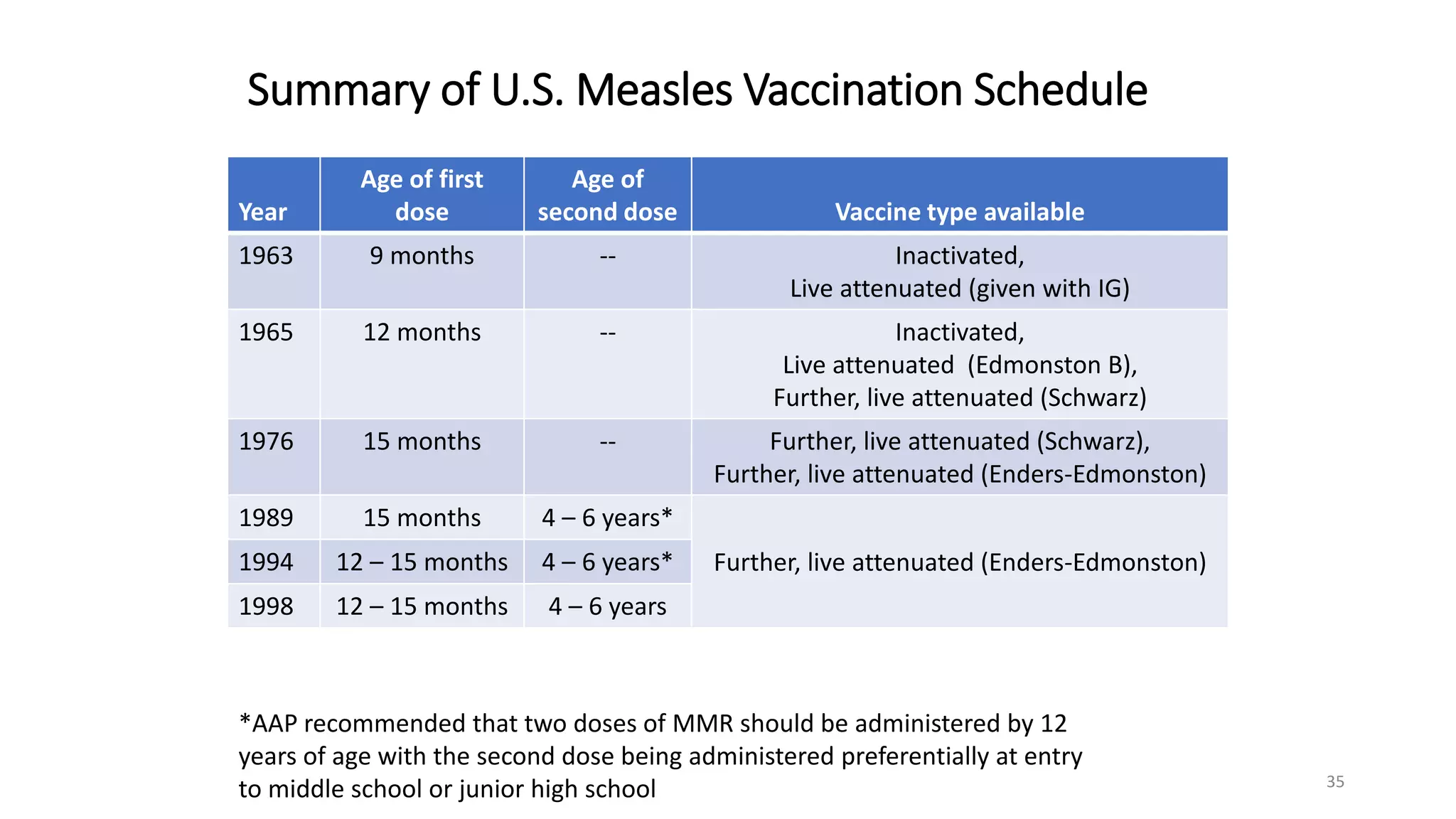
The document provides an extensive overview of measles, covering its pathogenesis, clinical manifestations, complications, and preventive measures. It highlights the importance of vaccination, the epidemiology of measles in the U.S., and the history of vaccination programs, emphasizing the role of two-dose vaccination schedules in achieving high immunity rates. Additionally, it addresses the severe impacts of measles in special populations and outlines recommendations for post-exposure prophylaxis.






















![Contraindications
• A contraindication is a condition in a recipient that greatly increases
the chance of a serious adverse reaction.
• People with a contraindication for MMR vaccine should not receive
MMR vaccine, including anyone who—
• Had a severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a
vaccine component
• Has a known severe immunodeficiency (e.g., from hematologic and solid
tumors, receipt of chemotherapy, congenital immunodeficiency, or long-term
immunosuppressive therapy or patients with human immunodeficiency virus
[HIV] infection who are severely immunocompromised)
• Is pregnant
• Has a history of anaphylactic reactions to neomycin](https://image.slidesharecdn.com/cpfmeaslespresentationfinal-190613144514/75/Measles-and-its-prevention-Slideset-by-professor-Edwards-45-2048.jpg)