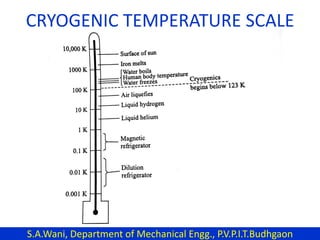
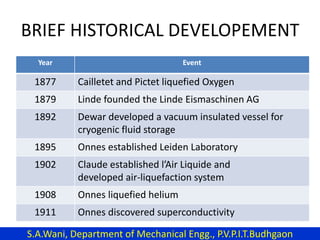
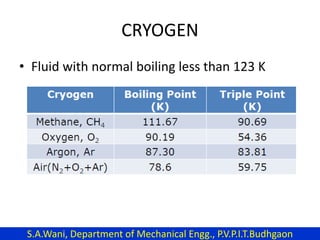
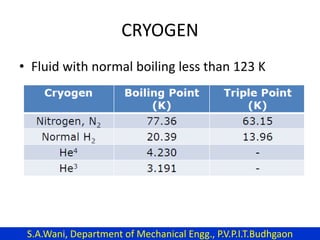
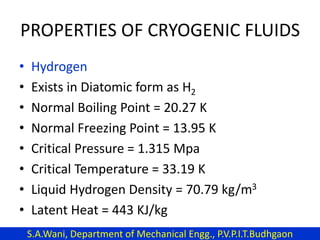
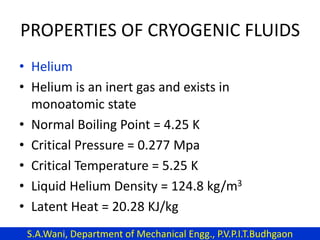
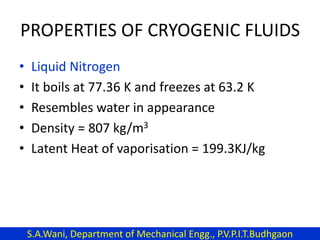
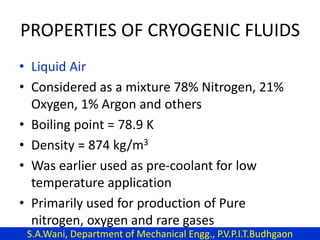
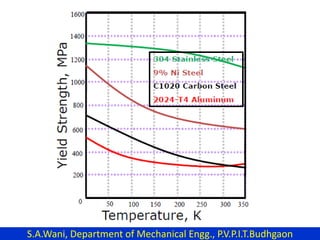
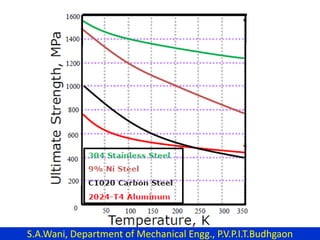
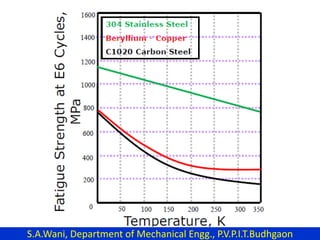
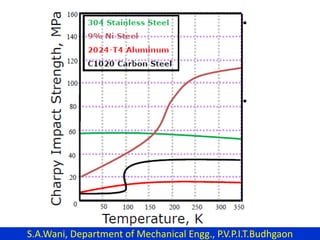
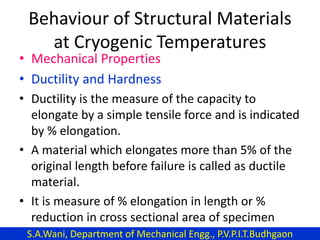
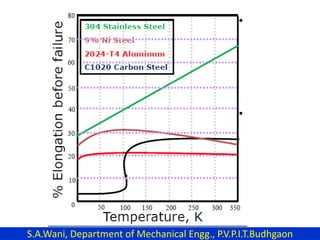
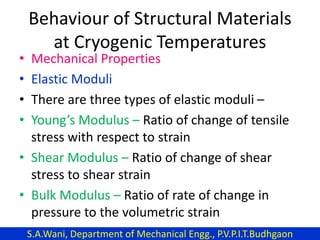
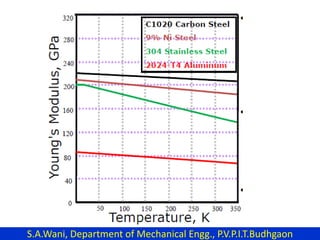
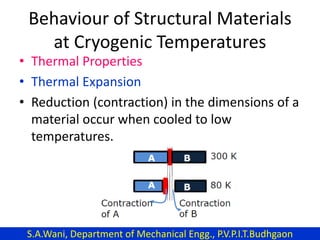
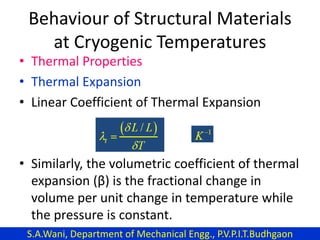
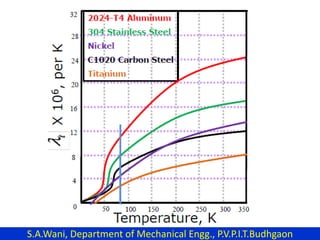
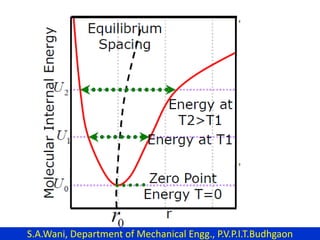
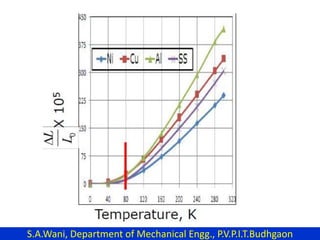
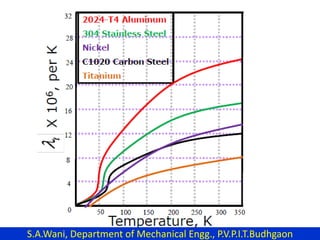
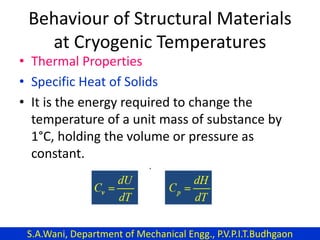
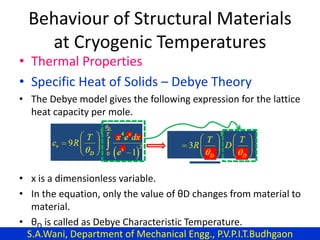
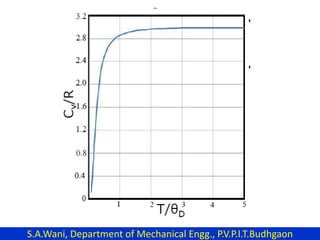
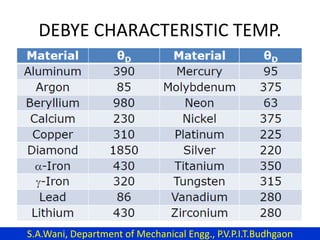
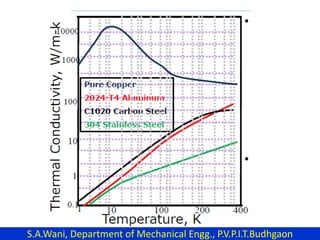
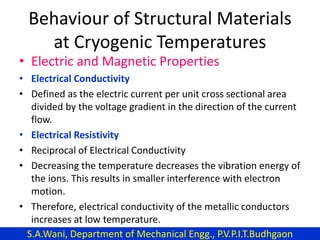
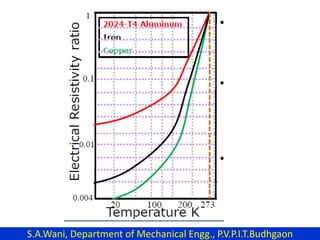
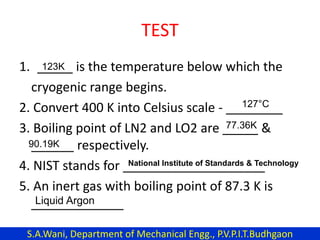
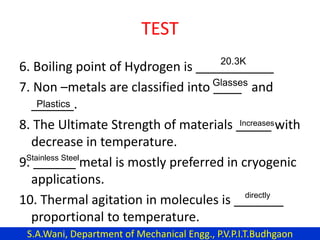
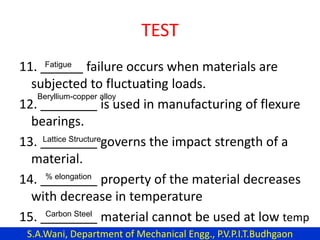
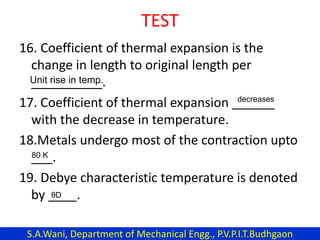
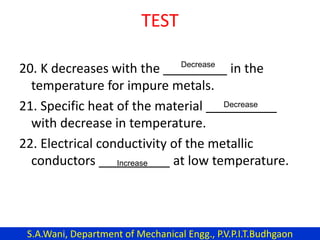
This document provides an introduction to cryogenics. It defines cryogenics as the science of producing low temperatures, below -150°C or 123 K. Cryogenics encompasses liquids such as nitrogen, oxygen, argon, hydrogen and helium. It has applications in fields like space studies, metallurgy, electronics, medicine and more. The document then reviews the history of cryogenics, including important discoveries and developments. It also provides details on common cryogenic fluids, their properties and typical applications.