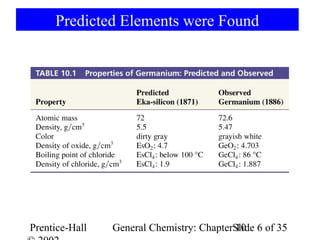
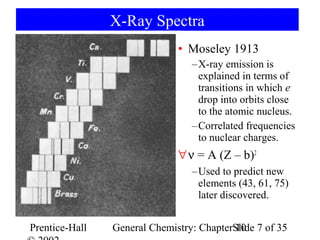
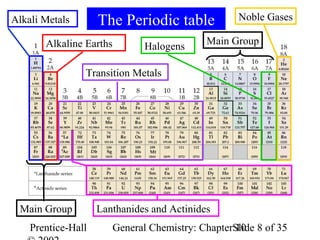
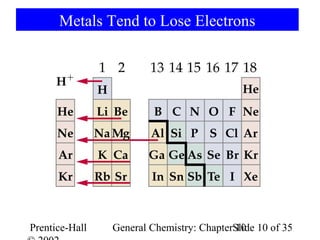
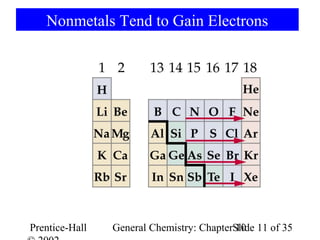
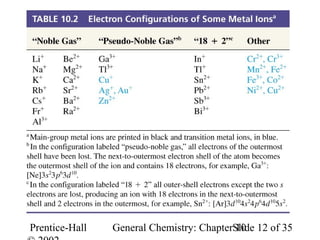
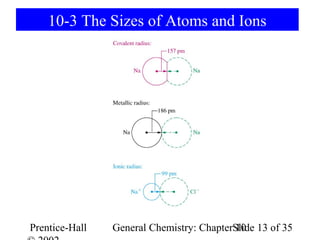
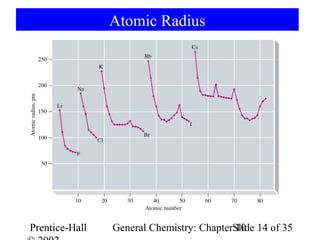
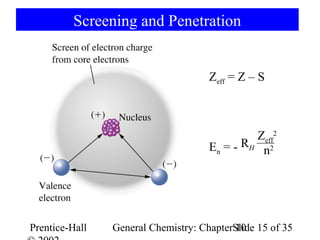
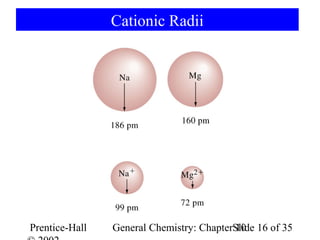
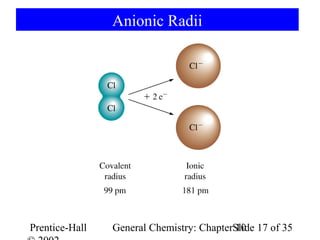
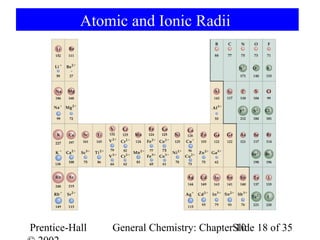
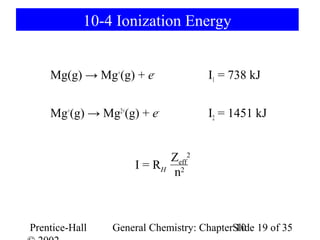
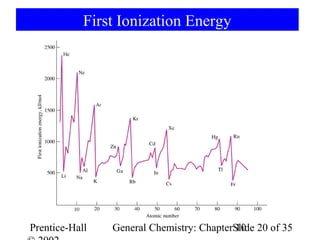
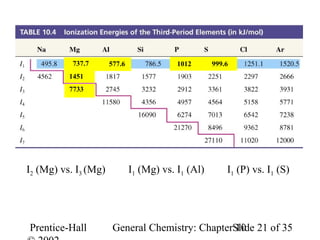
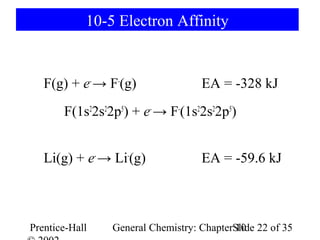
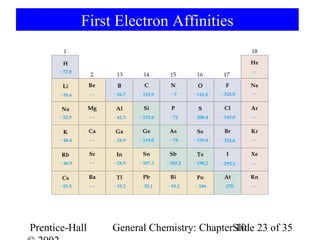
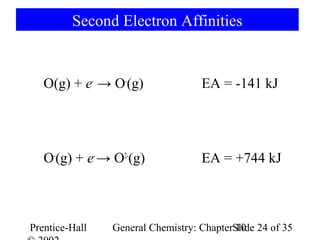
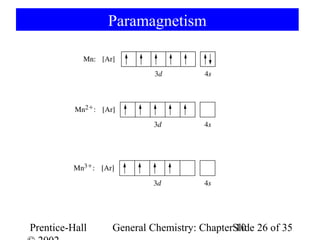
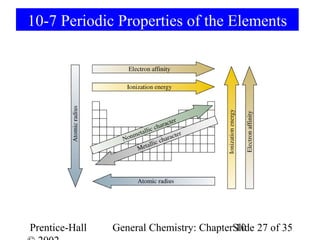
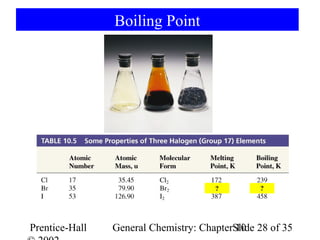
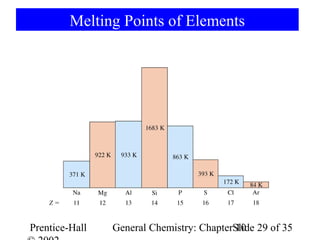
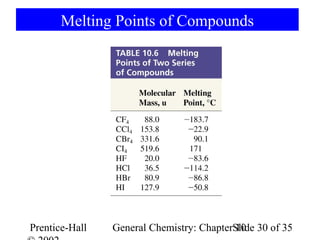
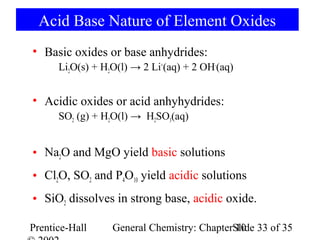
This document summarizes Chapter 10 from the textbook "General Chemistry: Principles and Modern Applications". The chapter discusses the periodic table and atomic properties. It covers the periodic law, classification of elements as metals and nonmetals, sizes of atoms and ions, ionization energy, electron affinity, and periodic trends in properties like melting points. The chapter also examines magnetic properties and how the periodic table can be used to predict trends in chemical behavior.