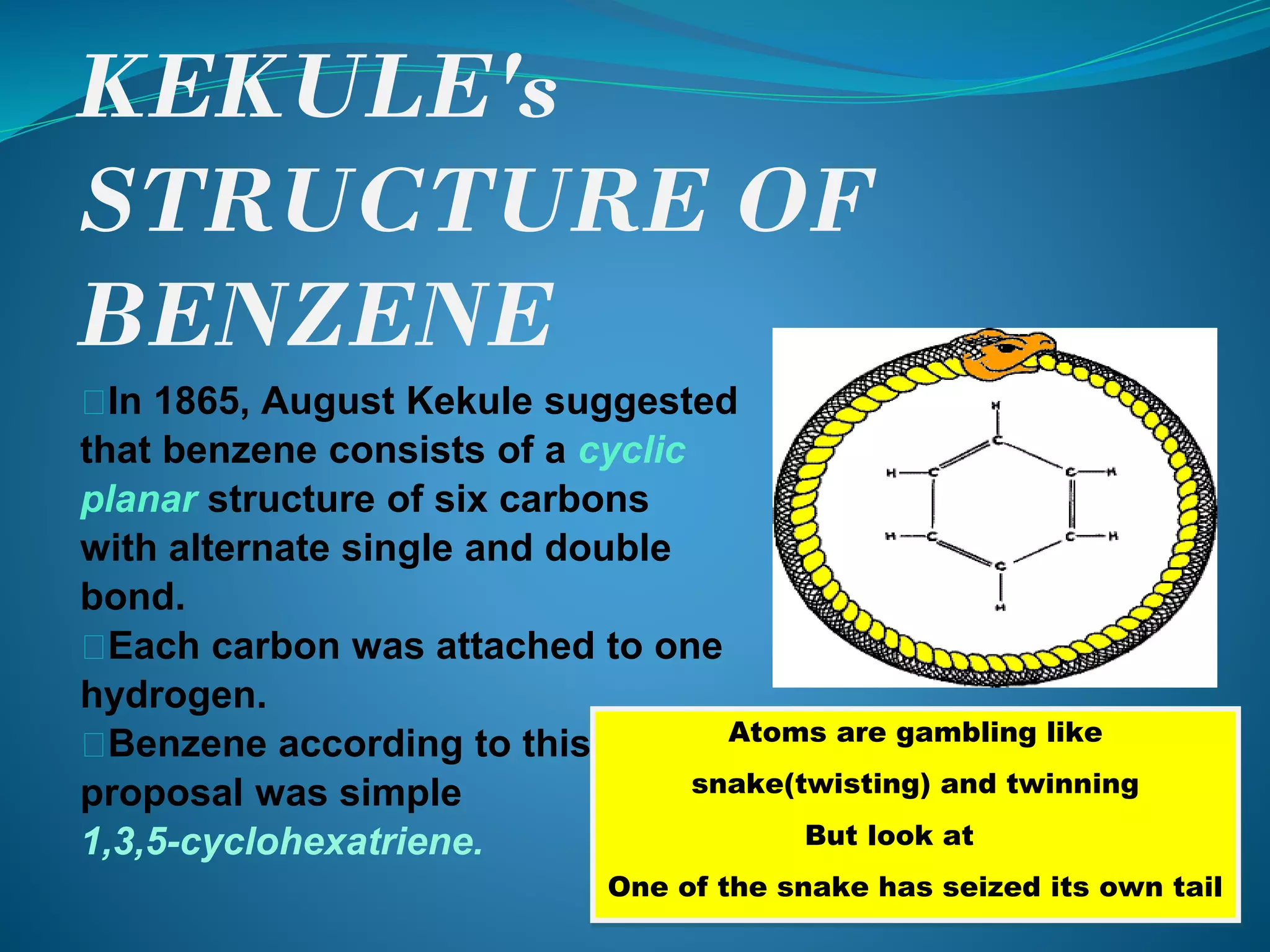
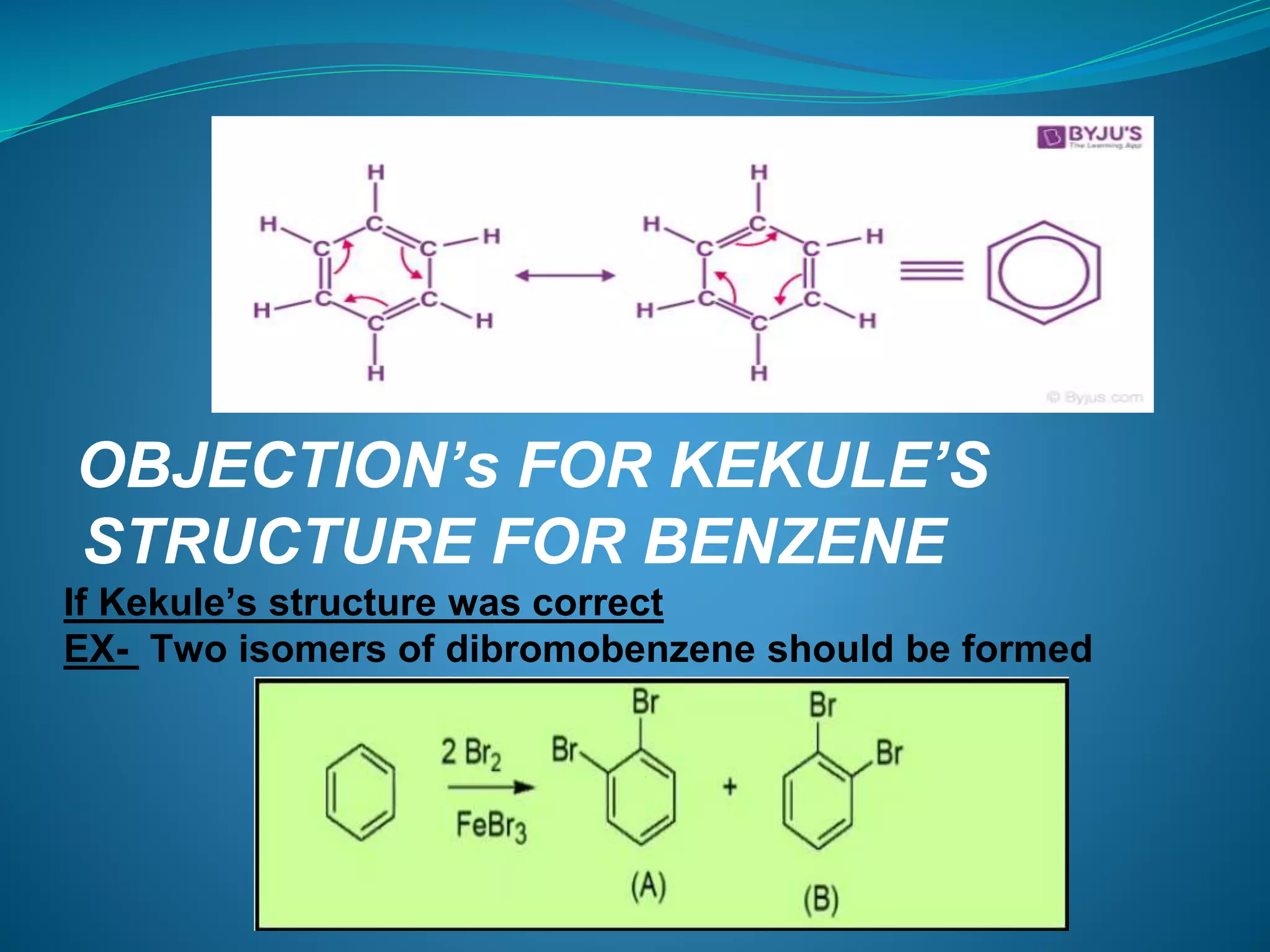
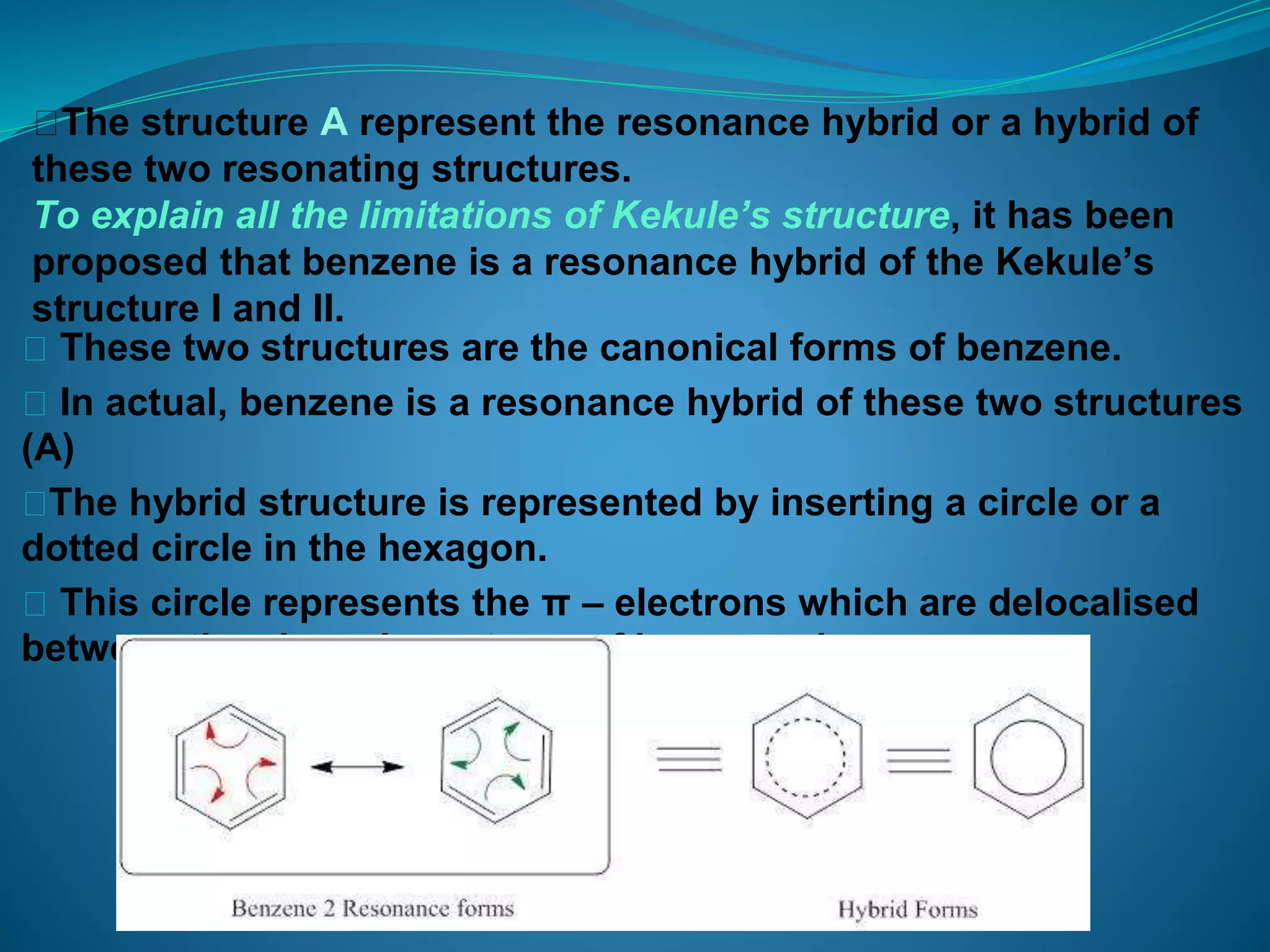
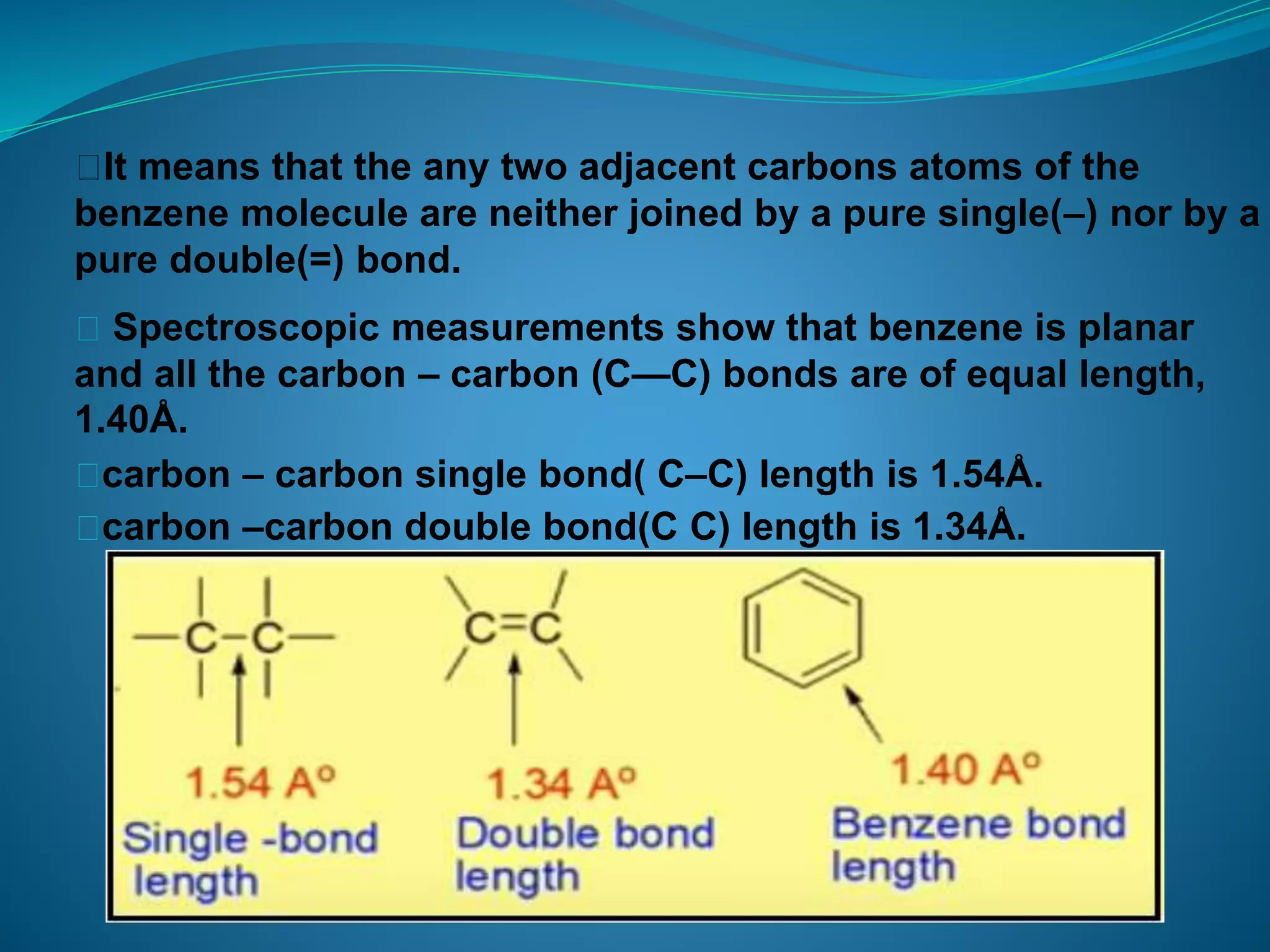
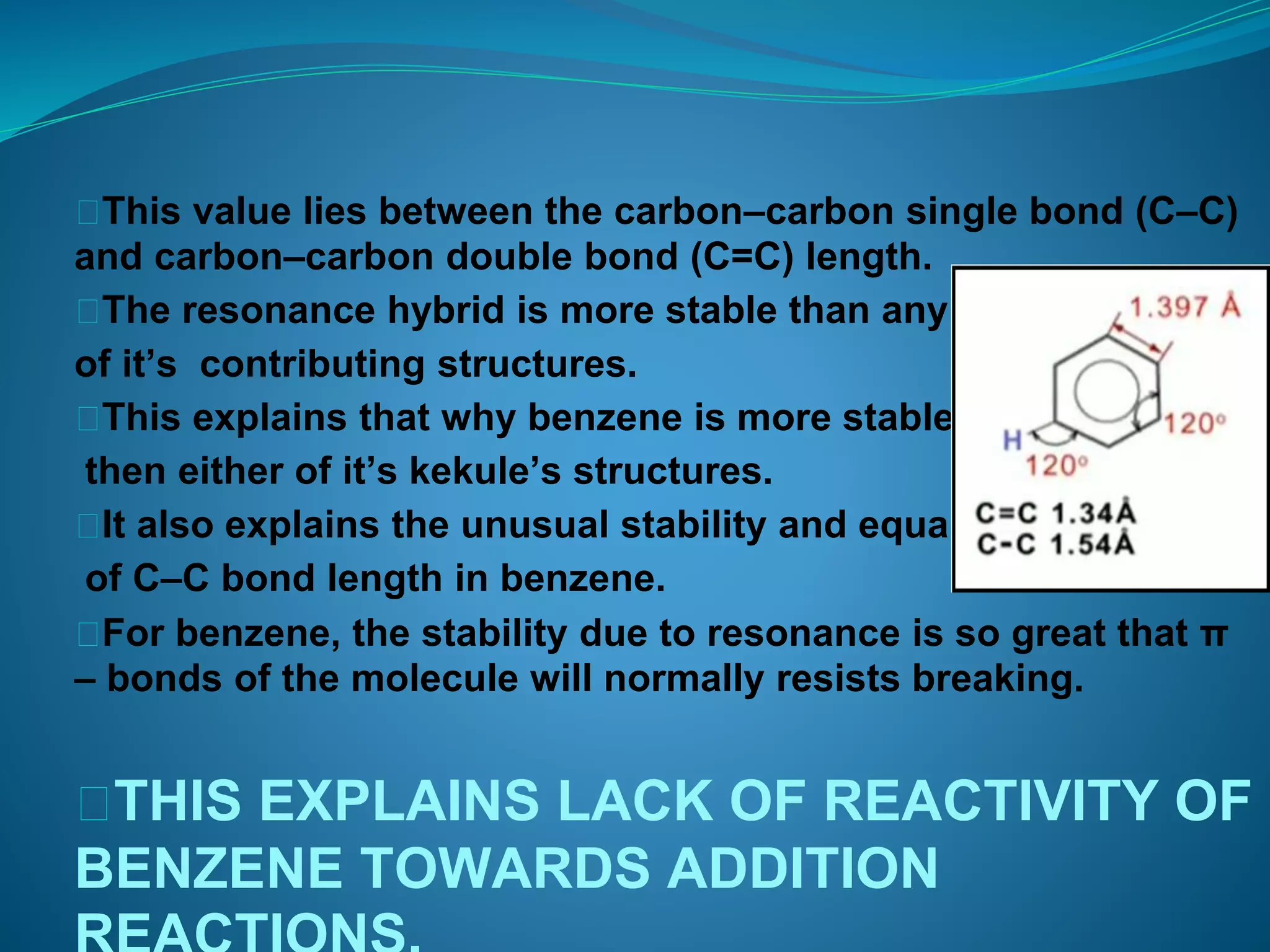
This document discusses the Kekulé structure of benzene proposed in 1865, highlighting its limitations such as inability to explain benzene's stability and its reaction patterns. It outlines the concept of resonance, explaining that benzene is better represented as a resonance hybrid of two contributing structures rather than a fixed structure with alternating single and double bonds. The resonance hybrid model accounts for benzene's uniform bond lengths and enhanced stability, clarifying why benzene resists typical reactions seen in unsaturated compounds.