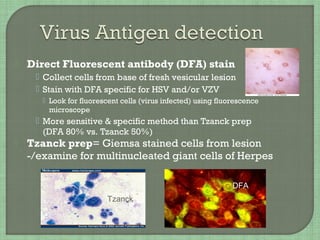
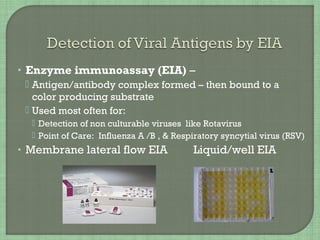
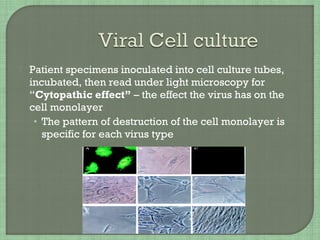
1. The document discusses various methods for diagnosing viral infections including direct fluorescent antibody staining, enzyme immunoassays, viral cell culture, and molecular amplification techniques.
2. It provides details on specific viruses that can be diagnosed by each method, such as herpes simplex virus diagnosed using direct fluorescent antibody staining or PCR.
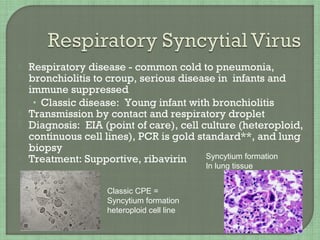
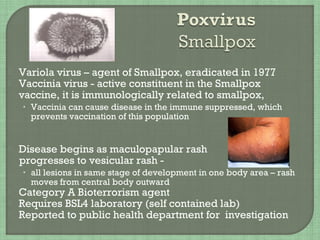
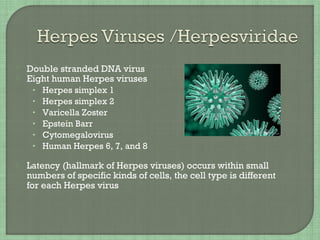
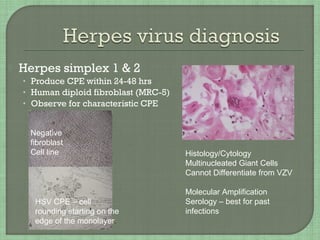
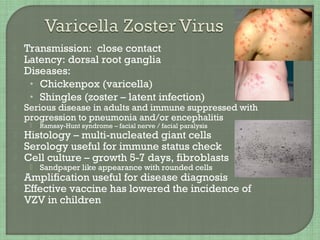
3. The document also summarizes the clinical presentation and pathogenesis of numerous viral infections including herpesviruses, adenoviruses, parvovirus B19, human papillomavirus, hepatitis viruses, arboviruses, and enteroviruses.








![ HH6
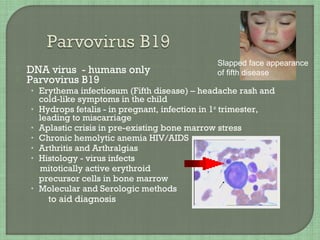
• Roseola [sixth disease]
• 6m-2yr high fever & rash
HH8
• (1) Kaposi’s sarcoma
• (2) Castleman disease
• (3) Primary effusion
lymphoma
Onion skin pattern of
Castleman disease
1
1
1
2
3](https://image.slidesharecdn.com/webpageviology2018-180410011923/85/Virology-2018-18-320.jpg)
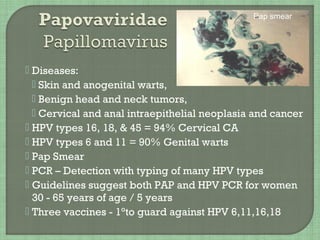
![• JC virus [John Cunningham]
Progressive multifocal leukoencephalopathy -
PML -Encephalitis of immune suppressed
Destroys oligodendrocytes in brain
• BK virus
Causes latent virus infection in kidney
Progression due to immune suppression
Hemorrhagic cystitis
• Histology
• PCR to aid diagnosis
Giant Glial Cells of JCV](https://image.slidesharecdn.com/webpageviology2018-180410011923/85/Virology-2018-23-320.jpg)












![ Disease: fever, malaise …. Death from respiratory
complications or secondary bacterial infection
Yearly H and N types dominate, most recently H1N1 and H3N2
Diagnosis
• Cell culture obsolete [RMK]
• Enzyme immunoassay (EIA) lateral flow membrane can be
used in point of care testing
• Amplification (PCR) gold standard for detection
Treatment: Amantadine and Tamiflu (Oseltamivir)
• Seasonal variation in susceptibility but Tamiflu sensitive
currently
Influenza B
• Milder form of Influenza like illness
• Usually <=10% of cases /year
Vaccinate – Trivalent vaccine -2 A viruses/1 B virus](https://image.slidesharecdn.com/webpageviology2018-180410011923/85/Virology-2018-39-320.jpg)
![Disease
• Fever, Rash, Dry Cough, Runny Nose,
Sore throat, inflamed eyes (photosensitive)
Can invade lung
• Respiratory spread - very contagious
• Koplik’s spot – bluish discoloration inner lining of
the cheek is pathognomonic
• Subacute sclerosing panencephalitis [SSPE]
Rare chronic degenerative neurological disease
Persistent infection with a mutated measles virus, due to
mutated virus there is total lack of an immune response
Diagnosis: Clinical symptoms, Serology , PCR
Vaccinate – MMR (Measles, Mumps, Rubella) vaccine
Treatment: Nothing specific, Immune globulin, vitamin A
H and E stain/ lung](https://image.slidesharecdn.com/webpageviology2018-180410011923/85/Virology-2018-40-320.jpg)