Rate of Reaction
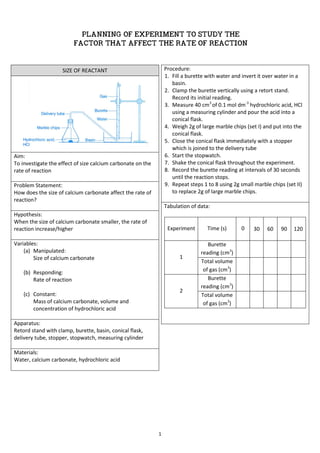
- 1. 1 SIZE OF REACTANT Aim: To investigate the effect of size calcium carbonate on the rate of reaction Problem Statement: How does the size of calcium carbonate affect the rate of reaction? Hypothesis: When the size of calcium carbonate smaller, the rate of reaction increase/higher Variables: (a) Manipulated: Size of calcium carbonate (b) Responding: Rate of reaction (c) Constant: Mass of calcium carbonate, volume and concentration of hydrochloric acid Apparatus: Retord stand with clamp, burette, basin, conical flask, delivery tube, stopper, stopwatch, measuring cylinder Materials: Water, calcium carbonate, hydrochloric acid Procedure: 1. Fill a burette with water and invert it over water in a basin. 2. Clamp the burette vertically using a retort stand. Record its initial reading. 3. Measure 40 cm3 of 0.1 mol dm-3 hydrochloric acid, HCl using a measuring cylinder and pour the acid into a conical flask. 4. Weigh 2g of large marble chips (set I) and put into the conical flask. 5. Close the conical flask immediately with a stopper which is joined to the delivery tube 6. Start the stopwatch. 7. Shake the conical flask throughout the experiment. 8. Record the burette reading at intervals of 30 seconds until the reaction stops. 9. Repeat steps 1 to 8 using 2g small marble chips (set II) to replace 2g of large marble chips. Tabulation of data: Experiment Time (s) 0 30 60 90 120 1 Burette reading (cm3 ) Total volume of gas (cm3 ) 2 Burette reading (cm3 ) Total volume of gas (cm3 ) PLANNING OF EXPERIMENT TO STUDY THE FACTOR THAT AFFECT THE RATE OF REACTION
- 2. 2 CONCENTRATION OF SOLUTION Aim: To investigate the effect of concentration sodium thiosulphate, Na₂S₂O₃ solution on the rate of reaction. Problem Statement: How does the concentration of sodium thiosulphate, Na₂S₂O₃ solution affect the rate of reaction? Hypothesis: The higher the concentration of sodium thiosulphate, Na₂S₂O₃ solution, the higher the rate of reaction. Variables: (a) Manipulated: Concentration of sodium thiosulphate, Na₂S₂O₃ solution (b) Responding: Rate of reaction (c) Constant: Volume and concentration of sulphuric acid. Apparatus: Conical flask, 50 cm³ measuring cylinder, 10 cm³ measuring cylinder, stopwatch Materials: 0.2 mol dm-3 sodium thiosulphate solution, 1.0 mol dm-3 sulphuric acid, distilled water, white paper marked ‘X’ at the centre Procedure: 1. Measure 50 cm3 of 0.2 mol dm-3 sodium thiosulphate, Na2S2O3 solution using a measuring cylinder and pour it into a conical flask. 2. Place the conical flask on top of a piece of white paper marked ‘X’. 3. Measure 5 cm3 of 1.0 mol dm-3 sulphuric acid, H2SO4 using another measuring cylinder. 4. Pour the sulphuric acid, H2SO4 quickly and carefully into the conical flask. At the same time, start the stopwatch. 5. Swirl the mixture in the conical flask. 6. Observe the ‘X’ mark vertically from the top of the conical flask through the solution. 7. Stop the stopwatch once the ‘X’ mark disappears from sight and record the time taken. 8. Repeat steps 1 to 7 using different concentrations of sodium thiosulphate, Na2S2O3 solution. Tabulation of data: Experiment Concentrations of sodium thiosulphate, Na2S2O3 solution (mol dm-3 ) Time taken for ‘X’ mark to disappear from sight, t (s) 1 2 3 4 5
- 3. 3 TEMPERATURE Aim: To investigate the effect of temperature sodium thiosulphate solution on the rate of reaction. Problem Statement: How does the temperature sodium thiosulphate solution affect the rate of reaction? Hypothesis: The higher the temperature sodium thiosulphate solution, the higher the rate of reaction. Variables: (a) Manipulated: Temperature of sodium thiosulphate solution (b) Responding: Rate of reaction (c) Constant: Volume and concentration of sodium thiosulphate solution, volume and concentration of sulphuric acid. Apparatus: Conical flask, measuring cylinder, stopwatch, thermometer, Bunsen burner, tripod stand, wire gauze. Materials: 0.2 mol dm-3 sodium thiosulphate solution, 1 mol dm-3 sulphuric acid, white paper marked ‘X’ at the centre Procedure: 1. Measure 50 cm3 of 0.2 mol dm-3 sodium thiosulphate, Na2S2O3 solution using a measuring cylinder and pour it into a conical flask. 2. Place the conical flask on top of a piece of white paper marked ‘X’. 3. Measure 5 cm3 of 1.0 mol dm-3 sulphuric acid, H2SO4 using another measuring cylinder. 4. Pour the sulphuric acid, H2SO4 quickly and carefully into the conical flask. At the same time, start the stopwatch. 5. Swirl the mixture in the conical flask. 6. Observe the ‘X’ mark vertically from the top of the conical flask through the solution. 7. Stop the stopwatch once the ‘X’ mark disappears from sight and record the time taken. 8. Repeat steps 1 to 7 using 50 cm3 of 0.2 mol dm-3 sodium thiosulphate, Na2S2O3 solution at 40°C, 50 °C, 60°C and 70°C by heating the solution before adding in 5 cm3 of 1.0 mol dm-3 sulphuric acid, H2SO4. Tabulation of data: Experiment Temperature (°C) Time taken for ‘X’ mark to disappear from sight, t (s) 1 28 2 40 3 50 4 60 5 70
- 4. 4 CATALYST [DECOMPOSITION OF HYDROGEN PEROXIDE] Aim: To investigate the effect of manganese(IV) oxide on the rate of decomposition of hydrogen peroxide. Problem Statement: Does the presence of manganese(IV) oxide affect the rate of decomposition of hydrogen peroxide? Hypothesis: The presence of manganese(IV) oxide will increase the rate of decomposition of hydrogen peroxide. Variables: (a) Manipulated: Presence of manganese(IV) oxide (b) Responding: Rate decomposition of hydrogen peroxide (c) Constant: Volume and concentration of hydrogen peroxide, mass of manganese(IV) oxide Apparatus: Retord stand with clamp, burette, basin, conical flask, delivery tube, stopper, stopwatch, measuring cylinder Materials: Water, hydrogen peroxide solution, manganese(IV) oxide Procedure: 1. Fill a burette with water and invert it over water in a basin. 2. Clamp the burette vertically using a retort stand. Record its initial reading. 3. Measure 25 cm3 of hydrogen peroxide, H2O2 using a measuring cylinder and pour into a conical flask. 4. Close the conical flask immediately with a stopper which is joined to the delivery tube 5. Start the stopwatch. 6. Shake the conical flask throughout the experiment. 7. Record the burette reading at intervals of 30 seconds until the reaction stops. 8. Repeat steps 1 to 7 by adding [1-5] g manganese(IV) oxide into the hydrogen peroxide solution. Tabulation of data: Experiment Time (s) 0 30 60 90 120 1 Burette reading (cm3 ) Total volume of gas (cm3 ) 2 Burette reading (cm3 ) Total volume of gas (cm3 )
- 5. 5 CATALYST [ZINC AND SULPHURIC ACID] Aim: To investigate the effect of copper(II) sulphate on the rate of reaction between zinc and sulphuric acid. Problem Statement: Does the presence of copper(II) sulphate affect the rate of reaction between zinc and sulphuric acid? Hypothesis: The presence of copper(II) sulphate will increase the rate of reaction between zinc and sulphuric acid. Variables: (d) Manipulated: Presence of copper(II) sulphate solution (e) Responding: Rate of reaction (f) Constant: Volume and concentration of sulphuric acid, mass of zinc Apparatus: Retord stand with clamp, burette, basin, conical flask, delivery tube, stopper, stopwatch, measuring cylinder Materials: Water, hydrogen peroxide solution, manganese(IV) oxide Procedure: 1. Fill a burette with water and invert it over water in a basin. 2. Clamp the burette vertically using a retort stand. Record its initial reading. 3. Measure 25 cm3 of 0.1 mol dm-3 sulphuric acid using a measuring cylinder and pour into a conical flask. 4. Close the conical flask immediately with a stopper which is joined to the delivery tube 5. Start the stopwatch. 6. Shake the conical flask throughout the experiment. 7. Record the burette reading at intervals of 30 seconds until the reaction stops. 8. Repeat steps 1 to 7 by adding a few drops of copper(II) sulphate solution into the mixture of zinc and sulphuric acid. Tabulation of data: Experiment Time (s) 0 30 60 90 120 1 Burette reading (cm3 ) Total volume of gas (cm3 ) 2 Burette reading (cm3 ) Total volume of gas (cm3 )