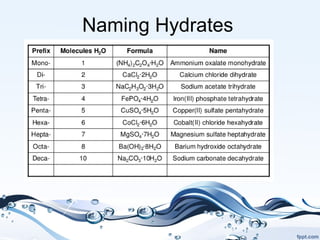
This document discusses hydrates, which are compounds that have water molecules bound to their atoms. Hydrates can be named using the compound name followed by a dot and the number of water molecules. The water molecules can be removed through heating, changing the hydrate into its anhydrous form. To determine the formula of a hydrate, the moles of water lost upon heating are calculated and compared to the moles of the compound to obtain a molar ratio, which is used to derive the chemical formula. Examples are provided to demonstrate solving for the formula and name of hydrates. Finally, some common uses of hydrates as drying agents and in solar energy are mentioned.