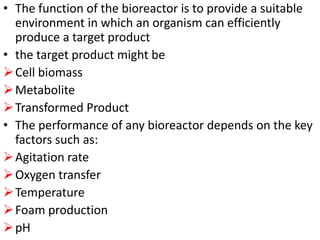
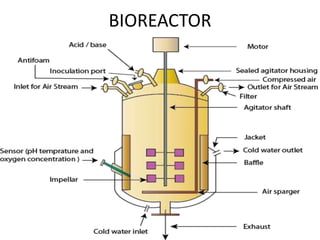
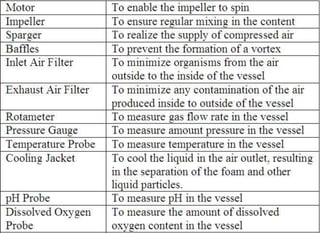
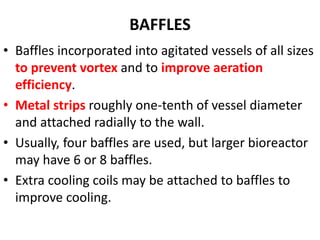
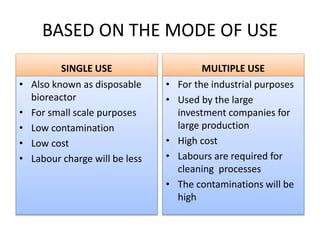
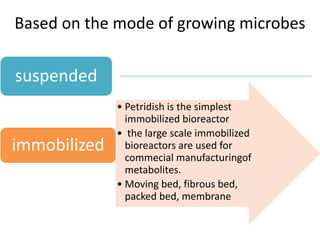
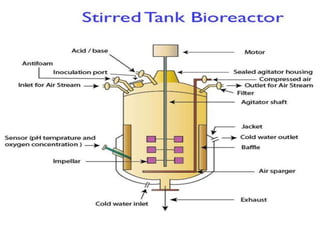
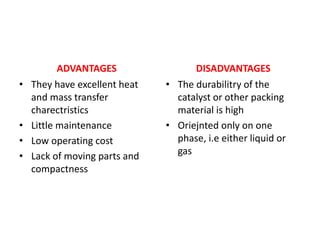
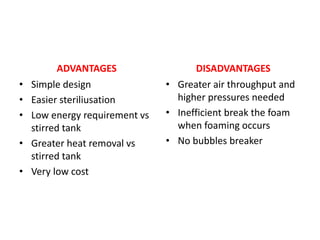
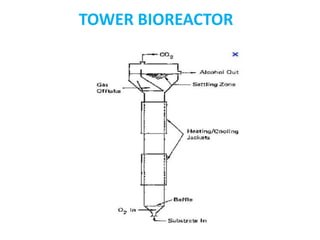
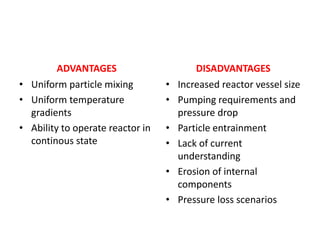
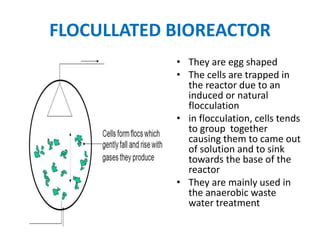
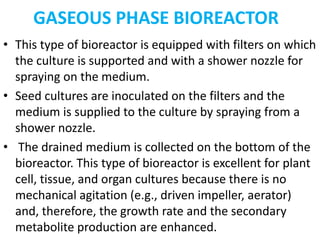
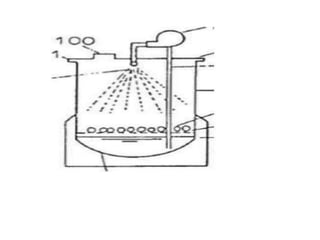
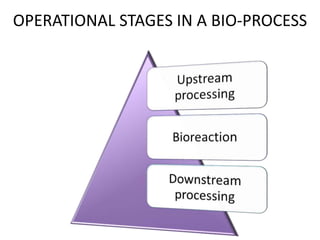
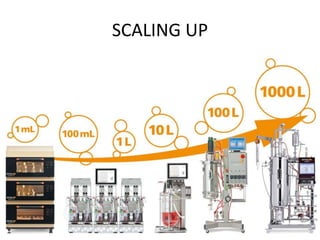
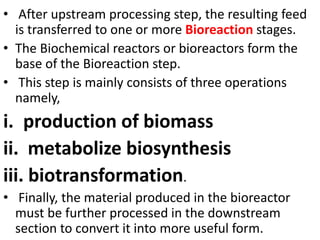
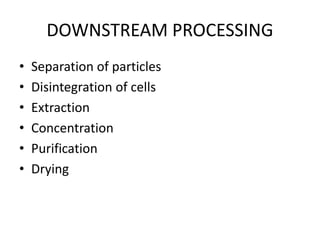
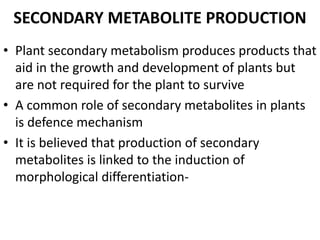
The document discusses bioreactors and various aspects related to their use and design. It describes bioreactors as vessels that provide a controlled environment for optimal growth and product formation of cell cultures. Various types of bioreactors are discussed based on factors like oxygen need, mode of use, operation, and type of microbial growth. Key components like vessels, agitation, aeration, and their functions are summarized. Applications in secondary metabolite production and downstream processing are also mentioned.